Abstract
Background
Advanced breast cancer remains clinically challenging due to its resistance to chemotherapy. To understand the underlying mechanisms of resistance and identify drugable target, the involvement of ceramide metabolism is investigated.
Methods
Ceramide levels in breast cancer tissues derived from 30 patients with stage IV breast cancer before and after chemotherapy were analyzed using liquid chromatography mass spectrometry. mRNA and protein levels of ceramide enzymes were examined using western blot and QRT-PCR. The effects of ceramide analog were investigated using cellular assays and xenograft tumor model.
Results
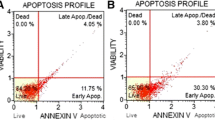
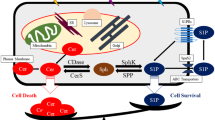
The results demonstrated that pro-apoptotic ceramide was significantly lower in all patients after chemotherapy, suggesting that downregulation of ceramide is a common feature of breast cancer patients in response to chemotherapy. Molecular characteristics analysis of ceramide indicated C16:0 as the predominant sphingolipid regulated by chemotherapy in breast cancer patients. Mechanistically, ceramide levels were suppressed by chemotherapy via increasing mRNA and protein levels of UDP-glucose ceramide glucosyltransferase (UGCG). Importantly, inhibition of UGCG using siRNA or upregulation of cellular ceramide levels using C2 ceramide alone inhibited proliferation and induced apoptosis of breast cancer cells, and enhanced the inhibitory effects of chemotherapeutic drugs in vitro and in vivo.
Conclusions
This study clearly demonstrated that the decreased ceramide production via up-regulating UGCG was involved in the resistance of breast cancer cells to chemotherapy. Stimulating ceramide or decreasing UGCG can potentially be useful for breast cancer treatment.




Similar content being viewed by others
References
Gradishar WJ (2016) Treatment challenges for community oncologists treating postmenopausal women with endocrine-resistant, hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Cancer Manag Res 8:85–94. doi:10.2147/CMAR.S98249
Videira M, Reis RL, Brito MA (2014) Deconstructing breast cancer cell biology and the mechanisms of multidrug resistance. Biochim Biophys Acta 1846(2):312–325. doi:10.1016/j.bbcan.2014.07.011
Sebens S, Schafer H (2012) The tumor stroma as mediator of drug resistance—a potential target to improve cancer therapy? Curr Pharm Biotechnol 13(11):2259–2272
Lee JJ, Loh K, Yap YS (2015) PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med 12 (4):342–354. doi:10.7497/j.issn.2095-3941.2015.0089
Wang T, Seah S, Loh X, Chan CW, Hartman M, Goh BC, Lee SC (2015) Simvastatin-induced breast cancer cell death and deactivation of PI3K/Akt and MAPK/ERK signalling are reversed by metabolic products of the mevalonate pathway. Oncotarget. doi:10.18632/oncotarget.6304
Ekiz HA, Baran Y (2010) Therapeutic applications of bioactive sphingolipids in hematological malignancies. Int J Cancer 127(7):1497–1506. doi:10.1002/ijc.25478
Bartke N, Hannun YA (2009) Bioactive sphingolipids: metabolism and function. J Lipid Res 50 Suppl:S91–S96. doi:10.1194/jlr.R800080-JLR200
Casson L, Howell L, Mathews LA, Ferrer M, Southall N, Guha R, Keller JM, Thomas C, Siskind LJ, Beverly LJ (2013) Inhibition of ceramide metabolism sensitizes human leukemia cells to inhibition of BCL2-like proteins. PLoS One 8(1):e54525. doi:10.1371/journal.pone.0054525
Wang J, Hu J, Jin Z, Wan H (2016) The sensitivity of chronic myeloid leukemia CD34 cells to Bcr-Abl tyrosine kinase inhibitors is modulated by ceramide levels. Leuk Res 47:32–40. doi:10.1016/j.leukres.2016.05.010
Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, Tang Y, Beckman BS (2004) Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther 309(2):523–532. doi:10.1124/jpet.103.062760
Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH (2009) Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res 50(8):1692–1707. doi:10.1194/jlr.D800051-JLR200
Diaz-Font A, Chabas A, Grinberg D, Vilageliu L (2006) RNAi-mediated inhibition of the glucosylceramide synthase (GCS) gene: A preliminary study towards a therapeutic strategy for Gaucher disease and other glycosphingolipid storage diseases. Blood cells molecules diseases 37(3):197–203. doi:10.1016/j.bcmd.2006.07.002
Pewzner-Jung Y, Ben-Dor S, Futerman AH (2006) When do lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem 281(35):25001–25005. doi:10.1074/jbc.R600010200
Hannun YA, Obeid LM (2011) Many ceramides. J Biol Chem 286(32):27855–27862. doi:10.1074/jbc.R111.254359
Tokuda N, Numata S, Li X, Nomura T, Takizawa M, Kondo Y, Yamashita Y, Hashimoto N, Kiyono T, Urano T (2013) β4GalT6 is involved in the synthesis of lactosylceramide with less intensity than β4GalT5. Glycobiology 23(10):1175–1183
Ogretmen B, Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4(8):604–616. doi:10.1038/nrc1411
Galadari S, Rahman A, Pallichankandy S, Thayyullathil F (2015) Tumor suppressive functions of ceramide: evidence and mechanisms. Apoptosis 20(5):689–711. doi:10.1007/s10495-015-1109-1
Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, Cabot MC (1998) Glucosylceramide: a marker for multiple-drug resistant cancers. Anticancer Res 18(1B):475–480
Schwamb J, Feldhaus V, Baumann M, Patz M, Brodesser S, Brinker R, Claasen J, Pallasch CP, Hallek M, Wendtner CM, Frenzel LP (2012) B-cell receptor triggers drug sensitivity of primary CLL cells by controlling glucosylation of ceramides. Blood 120(19):3978–3985. doi:10.1182/blood-2012-05-431783
Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC (2007) Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta 1771(12):1407–1417. doi:10.1016/j.bbalip.2007.09.005
Schiffmann S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, Kaufmann M, Ackermann H, Lotsch J, Schmidt H, Geisslinger G, Grosch S (2009) Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis 30(5):745–752. doi:10.1093/carcin/bgp061
Kanto T, Kalinski P, Hunter OC, Lotze MT, Amoscato AA (2001) Ceramide mediates tumor-induced dendritic cell apoptosis. J Immunol 167(7):3773–3784
Senchenkov A, Litvak DA, Cabot MC (2001) Targeting ceramide metabolism–a strategy for overcoming drug resistance. J Natl Cancer Inst 93(5):347–357
Maguer-Satta V (1998) CML and apoptosis: the ceramide pathway. Hematol Cell Ther 40(5):233–236
Nica AF, Tsao CC, Watt JC, Jiffar T, Kurinna S, Jurasz P, Konopleva M, Andreeff M, Radomski MW, Ruvolo PP (2008) Ceramide promotes apoptosis in chronic myelogenous leukemia-derived K562 cells by a mechanism involving caspase-8 and JNK. Cell Cycle 7(21):3362–3370
Jaeger U (2012) Drug sensitivity and sphingolipid metabolism in CLL. Blood 120(19):3865–3866. doi:10.1182/blood-2012-09-455394
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81202095) and the Research Fund for the Doctoral Program of Higher Education of China (20120142120053).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interests.
Research involving human participants and/or animals
All procedures involving human participants were performed in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures involving animals were conducted according to the guidelines approved by the Institutional Animal Care and Use Committee of Tongji University.
Inform consent
Written informed consent was obtained from all individual participants included in the study under institutional review board approved protocols.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Che, J., Huang, Y., Xu, C. et al. Increased ceramide production sensitizes breast cancer cell response to chemotherapy. Cancer Chemother Pharmacol 79, 933–941 (2017). https://doi.org/10.1007/s00280-017-3292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3292-y