Abstract
Purpose
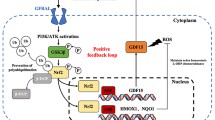
Nrf2 and its role in controlling levels of the AKR family of aldo–keto reductases which have been implicated in resistance to platinum-based chemotherapy was studied in ovarian, cervical and lung cell lines.
Methods
Nrf2 shRNA knockdowns of cells from different tumor origins were prepared to determine the role of this factor in producing resistance to platinum chemotherapy.
Results
Nrf2 knockdowns resulted in marked decreases in AKR1C1, AKR1C2 and to a lesser extent AKR1C3. Additionally, all other candidate enzymes GSTπ and TRX1 were decreased, but their role was difficult to correlate to cytotoxicity. Nrf2 knockdowns exhibited marked increases in mitochondrial membrane depolarization and ROS production following cisplatin treatment, with the cervical ME180R knockdowns exhibiting the greatest effect (AKR1C1 and AKR1C2 levels were decreased in the ME180R and SKOV3 cells to near zero). Oxaliplatin tended to parallel cisplatin, except it markedly stimulated O2 − production not \( {\text{H}}_{2} {\text{O}}_{2}\) by oxaliplatin treatment of the ME180R cells. The pJNK/p38 pathway has been implicated in cisplatin cytotoxicity, and significant phosphorylation of pJNK was observed in the SKOV3 and ME180R and p38 in the SKOV3 knockdowns. Phosphorylation of ATF2 was decreased in the Nrf2 knockdowns (Crf38, Srf6, Arf5) which could affect its interaction with JNK and p38. Oxaliplatin treatment showed minimal effects on the JNK/p38 pathway, showing that its mode of action is different although ROS generation appeared an initial step with both drugs.
Conclusions
Nrf2 controls a multitude of different candidate genes; however, it did markedly modulate cisplatin resistance through the AKR family. This involved ROS production and activation of the pJNK/p38 pathway with involvement of ATF2.
Graphical Abstract







Similar content being viewed by others
References
Kansanen E, Jyrkkanen HK, Levonen AL (2012) Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic Biol Med 52:973–982
Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M (2004) Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci 101:2046–2051
Baird L, Swift S, Lleres D, Dinkova-Kostova AT (2014) Monitoring Keap1-Nrf2 interactions in single live cells. Biotechnol Adv 32:1133–1144
Niture SK, Khatri R, Jaiswal AK (2014) Regulation of Nrf2-an update. Free Radic Biol Med 66:36–44
Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C et al (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acid Res 38:5718–5734
van der Wijst MGP, Brown R, Rots MG (2014) Nrf2, the master redox switch: the Achilles’ heel of ovarian cancer? Biochim Biophys Acta 1846:494–509
Konstantinopoulos PA, Spentzos D, Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP et al (2011) Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res 71:5081–5089
Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A et al (2012) Transcriptomic and proteomic profiling of Keap1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat 132:175–187
Hanada N, Takahata T, Zhou Q, Ye X, Sun R, Itoh J et al (2012) Methylation of the Keap1 gene promoter region in human colorectal cancer. BMC Cancer 12:1–11
Loignon M, Miao W, Hu L, Bier A, Bismar TA, Scrivens PJ et al (2012) Cul3 overexpression depletes Nrf2 in breast cancer and is associated with sensitivity to carcinogens, to oxidative stress, and to chemotherapy. Mol Cancer Ther 8:2432–2440
Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J et al (2008) RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68:7975–7984
Shim G-S, Manandhar S, Shin D-H, Kim T-H, Kwak M-K (2009) Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of NRF2 pathway. Free Radic Biol Med 347:1619–1631
Manandhar S, Choi B-H, Jung K-A, Ryoo I-G, Song M, Kang SJ et al (2012) NRF2 inihibition represses ErbB2 signaling in ovarian carcinoma cells: implications for tumor growth retardation and docetaxel sensitivity. Free Radic Biol Med 52:1773–1785
Chen C-C, Chu C-B, Liu K-J, Huang C-YF, Chang J-Y, Pan W-Y et al (2013) Gene expression profiling for analysis acquired oxaliplatin resistant factors in human gastric carcinoma TSGH-S3 cells: the role of IL-6 signaling and Nrf2/AKR1C axis identification. Biochem Pharmacol 28:872–887
Hsu NY, Ho HC, Chow KC, Lin TY, Shih CS, Wang LS, Tsai CM (2001) Overexpression of dihydrodiol dehydrogenase as a prognostic marker of non-small cell lung cancer. Cancer Res 61:2727–2731
Kuang P, Zhou C, Li X, Ren S, Li B, Wang Y et al (2012) Proteomics-based identification of secreted protein dihydrodiol dehydrogenase 2 as a potential biomarker for predicting cisplatin efficacy in advanced NSCLC patients. Lung Cancer 77:427–432
Smithgall TE, Harvey RG, Penning TM (1986) Regio-and stereospecificity of homogeneous 3 alpha hydroxysteroid-dihydrodiol dehydrogenase for transdihydrodiol metabolites of polycyclic aromatic hydrocarbons. J Biol Chem 261:6184–6191
Burczynski ME, Sridhar GR, Palackal NT, Penning TM (2001) The reactive oxygen species-and michael acceptor—inducible human aldo-keto reductase AKR1C1 reduces the α, β-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-noene. J Biol Chem 276:2890–2897
Deng HB, Adikari M, Parekh HK, Simpkins H (2002) Increased expression of dihydrodiol dehydrogenase induces resistance to cisplatin in human ovarian carcinoma cells. J Biol Chem 277:15035–15043
Deng HB, Adikari M, Parekh HK, Simpkins H (2004) Ubiquitous induction of resistance to platinum drugs in human ovarian, cervical, germ cell and lung carcinoma tumor cells overexpressing isoforms 1 and 2 of dihydrodriol dehydrogenase. Cancer Chemother Pharmacol 54:301–307
Chen J, Emara N, Solomides C, Parekh H, Simpkins H (2010) Resistance to platinum based chemotherapy in lung cancer cell lines. Cancer Chem Pharm 66(1):103–1111
Chen J, Parekh H, Solomides C, Simpkins F, Simpkins H (2015) Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother Pharmacol 75:1217–1227
Takada E, Hata K, Mizuguchi J (2008) C-Jun-NH2-terminal kinase potentiates apoptotic cell death in response to carboplatin in B lymphoma cells. Cancer Chemother Pharmacol 62:569–576
Bragado P, Armesilla A, Silva A, Porras A (2007) Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis 12:1733–1742
Rao DD, Vorhies JS, Senzer N, Nemunaitis J (2009) siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev 61:746–759
Hayakawa J, Depatie C, Ohmichi M, Mercola D (2003) The activation of c-Jun NH2- terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J Biol Chem 278:20562–20592
Korsch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA et al (2012) DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification redundancy and contamination. Gynecol Oncol 127:241–248
Yokomizo A, Ono M, Nanri H, Makino Y, Ohga T, Wada M et al (1995) Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin and ectoposide. Cancer Res 55:4293–4296
Sasada T, Iwata S, Sato N, Kitaoka Y, Hirota K, Nakamura K et al (1996) Redox control of resistance to CDDP: protective effect of human thioredoxin against CDDP-induced cytotoxicity. J Clin Invest 91:2268–2276
Yamada M, Tomida A, Yoshikawa H, Taketani Y, Tsuruo T (1997) Overexpression of thioredoxin does not confer resistance to cisplatin in transfected human ovarian and colon cancer cell lines. Cancer Chemother Pharmacol 40:31–37
Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin J, Manketkom S (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiation Phys Chem 72:323–331
Camara AKS, Riess ML, Kevin LG, Novalia E, Stowe DL (2004) Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol (Heart Circ Physiol) 286:H1289–H1299
Gebauer A, Mirakhur B, Nguyen A, Shue SK, Simpkins H, Dhanasekaran N (2000) Cisplatin resistance involving the defective processing of MEKKI in human ovarian adenocarcinoma 2008/C13 cells. Int Jnl Oncol 16:321–325
Gupta S, Campbell D, Derijard B, Davis RJ (1995) Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267:389–393
Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22:7369–7375
Cullen KJ, Newkirk KA, Schumaker LM, Aldosari N, Rone JD, Haddad BR (2003) Glutathione-S-transferase π amplification is associated with cisplatin resistance in head and neck sqamous cell carcinoma cell lines and primary tumors. Cancer Res 63:8097–8102
Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M, Lage H et al. Augmented expression of metallothionein and glutathione-S-transferes π as unfavorable prognostic factors in cisplatin treated ovarian cancer patients Virchows Arch 447:626–633
Pasello M, Michelacci F, Scionti I, Hattinger CM, Zuntini M, Caccuri AM et al (2008) Overcoming glutathione-S-transferase P1-related cisplatin resistance in osteosarcoma. Cancer Re 68:6661–6668
De Luca A, Tregno FP, Sau A, Pastore A, Palumbo C, Alama A et al (2013) Glutathione S-transferase P1-1 as a target for mesothelioma treatment. Cancer Sci 104:223–230
Knippen S, Loning T, Muller V, Schroder C, Janicke F, Milde-Langosch K (2009) Expression and prognostic value of activating transcription factor 2 (ATF2) and its phosphorylated form in mammary carcinomas. Anticancer Res 29:183–190
Duffey D, Dolgilevich S, Razzouk S, Li L, Green R, Gorti GK (2011) Activating transcription factor-2 (ATF2) in survival mechanisms in head and neck carcinoma cells. Head Neck 33:1586–1599
Lo Iacono M, Monica V, Vavalàt T, Gisabella M, Saviozzi S, Bracco E et al (2015) ATF2 contributes to cisplatin resistance in non-small cell lung cancer and celastrol induces cisplatin resensitization through inhibition of JNK/ATF2 pathway. Int J Cancer 136:2598–2609
Lau E, Ronai Z (2012) ATF2—at the crossroad of nuclear and cytosolic functions. J Cell Sci 125:2815–2824
Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, Pendyla L (2011) In vitro studies on the mechanisms of oxaliplatin resistance. Cancer Chemother Pharmacol 48:398–406
Fink D, Zheng H, Nebel S, Norris PS, Aebi S, Lin TP et al (1997) In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res 57:1846–1847
Lee SH, Bahn JH, Whitlock NC, Baek SJ (2010) Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene 29:5182–5192
Brown SL, Sekhar KR, Rachakonda G, Sasi S, Freeman ML (2008) Activating transcription factor 3 is a novel repressor of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2)-regulated stress pathway. Cancer Res 68(2):364–368
Acknowledgements
The authors thank Diane Murphy for helping prepare the manuscript.
Funding
The work was supported by the Northwell Health System.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Chen, J., Solomides, C., Simpkins, F. et al. The role of Nrf2 and ATF2 in resistance to platinum-based chemotherapy. Cancer Chemother Pharmacol 79, 369–380 (2017). https://doi.org/10.1007/s00280-016-3225-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3225-1