Abstract
Background
Irinotecan plus S-1 (IRIS) is the only oral fluoropyrimidine-based regimen reported to be non-inferior to FOLFIRI and widely used in clinical practice for metastatic colorectal cancer (mCRC) patients. However, the combination of IRIS plus an anti-EGFR agent has not been evaluated previously. This study aimed to investigate the feasibility and efficacy of IRIS with panitumumab as second-line therapy for wild-type KRAS mCRC.
Methods
Main inclusion criteria were patients with wild-type KRAS mCRC refractory to one prior chemotherapy regimen for mCRC, ECOG PS 0–2, and age ≥20 years. Patients received panitumumab (6 mg/kg) and irinotecan (100 mg/m2) on days 1 and 15 and S-1 (40–60 mg according to body surface area) twice daily for 2 weeks, repeated every 4 weeks. The primary endpoint was the feasibility of the therapy. The secondary endpoints were response rate (RR), progression-free survival (PFS), and overall survival (OS).
Results
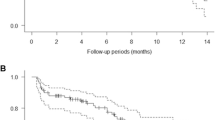
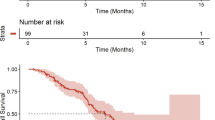
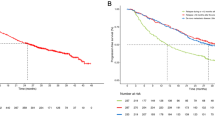
A total of 36 patients received protocol treatment in eight centers. Of these, 23 patients (63.9 %) completed protocol treatment, demonstrating achievement of the primary endpoint. The most frequent grade 3/4 toxicities were diarrhea (16.7 %), acne-like rash (13.9 %), and neutropenia (11.1 %). The overall RR was 33.3 % (12/36). Of these patients, five underwent conversion surgery. Median PFS and OS were 9.5 months (95 % CI 3.5–15.4 months) and 20.1 months (95 % CI 16.7–23.2 months), respectively.
Conclusion
IRIS plus panitumumab has an acceptable toxicity profile and a promising efficacy in patients with previously treated wild-type KRAS mCRC. Accordingly, this regimen can be an additional treatment option for second-line chemotherapy in wild-type KRAS mCRC.

Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, Group EGW (2014) Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii1–iii9. doi:10.1093/annonc/mdu260
Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237. doi:10.1200/JCO.2004.05.113
Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboüé R, Tuech JJ, Queuniet AM, Paillot B, Sabourin JC, Michot F, Michel P, Frebourg T (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96(8):1166–1169. doi:10.1038/sj.bjc.6603685
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26(10):1626–1634. doi:10.1200/JCO.2007.14.7116
De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19(3):508–515. doi:10.1093/annonc/mdm496
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28(31):4706–4713. doi:10.1200/JCO.2009.27.6055
Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, Yamaguchi K, Takiuchi H, Esaki T, Tokunaga S, Kuwano H, Komatsu Y, Watanabe M, Hyodo I, Morita S, Sugihara K (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11(9):853–860. doi:10.1016/S1470-2045(10)70181-9
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Komatsu Y, Yuki S, Sogabe S, Fukushima H, Nakatsumi H, Kobayashi Y, Iwanaga I, Nakamura M, Hatanaka K, Miyagishima T, Kudo M, Munakata M, Meguro T, Tateyama M, Sakata Y (2012) Phase II study of combined chemotherapy with irinotecan and S-1 (IRIS) plus bevacizumab in patients with inoperable recurrent or advanced colorectal cancer. Acta Oncol 51(7):867–872. doi:10.3109/0284186X.2012.682629
Komatsu Y, Ishioka C, Shimada K, Yamada Y, Gamoh M, Sato A, Yamaguchi T, Yuki S, Morita S, Takahashi S, Goto R, Kurihara M (2015) Study protocol of the TRICOLORE trial: a randomized phase III study of oxaliplatin-based chemotherapy versus combination chemotherapy with S-1, irinotecan, and bevacizumab as first-line therapy for metastatic colorectal cancer. BMC Cancer 15:626. doi:10.1186/s12885-015-1630-1
Cohn AL, Shumaker GC, Khandelwal P, Smith DA, Neubauer MA, Mehta N, Richards D, Watkins DL, Zhang K, Yassine MR (2011) An open-label, single-arm, phase 2 trial of panitumumab plus FOLFIRI as second-line therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer 10(3):171–177. doi:10.1016/j.clcc.2011.03.022
Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D (2008) Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19(10):1720–1726. doi:10.1093/annonc/mdn370
Köhne CH, De Greve J, Hartmann JT, Lang I, Vergauwe P, Becker K, Braumann D, Joosens E, Müller L, Janssens J, Bokemeyer C, Reimer P, Link H, Späth-Schwalbe E, Wilke HJ, Bleiberg H, Den Van, Brande J, Debois M, Bethe U, Van Cutsem E (2008) Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol 19(5):920–926. doi:10.1093/annonc/mdm544
Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Saltz L (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12):2006–2012. doi:10.1200/JCO.2007.14.9898
Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y, Sasaki Y, Satoh T, Takahashi K, Mishima H, Muro K, Watanabe M, Sakata Y, Morita S, Shimada Y, Sugihara K (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 14(13):1278–1286. doi:10.1016/S1470-2045(13)70490-X
Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke A, Schmiegel W, Schmoll HJ, Porschen R (2008) Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol 26(36):5910–5917. doi:10.1200/JCO.2008.16.7759
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25(30):4779–4786. doi:10.1200/JCO.2007.11.3357
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP, Investigators MCT (2011) Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377(9783):2103–2114. doi:10.1016/S0140-6736(11)60613-2
Ajani JA, Faust J, Ikeda K, Yao JC, Anbe H, Carr KL, Houghton M, Urrea P (2005) Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 23(28):6957–6965. doi:10.1200/JCO.2005.01.917
Ajani JA, Lee FC, Singh DA, Haller DG, Lenz HJ, Benson AB, Yanagihara R, Phan AT, Yao JC, Strumberg D (2006) Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24(4):663–667. doi:10.1200/JCO.2005.04.2994
Acknowledgments
We thank all patients, clinicians, and support stuff who participated in this study. We also thank Dr. Hiroaki Mikasa, Kimiko Fukuchi, and Michiko Yoshimaru for their advice and kind help. This study was partly supported by the research fund from Taiho Pharmaceutical Co. Ltd. The sponsor played no role in the design or conduct of the study; in the collection, management, analyses, or interpretation of data; or in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tetsuji Takayama has received research funding from Taiho Pharmaceutical Co. Ltd. All other authors declare that they have no conflict of interest relevant to this study.
Rights and permissions
About this article
Cite this article
Takaoka, T., Kimura, T., Shimoyama, R. et al. Panitumumab in combination with irinotecan plus S-1 (IRIS) as second-line therapy for metastatic colorectal cancer. Cancer Chemother Pharmacol 78, 397–403 (2016). https://doi.org/10.1007/s00280-016-3096-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3096-5