Abstract
Purpose
In a phase III study of gemcitabine plus erlotinib for advanced pancreatic cancer conducted in Canada, the incidence of interstitial lung disease (ILD) was 3.5 %. However, the incidence of ILD was reported as high as 8.5 % in a Japanese phase II study. These results suggest the influence of ethnic factors in the association of the use of gemcitabine plus erlotinib with the incidence of ILD. Here, we conducted a prospective study to analyze the relationship between human leukocyte antigen (HLA) alleles and ILD in Japanese patients with advanced pancreatic cancer receiving gemcitabine plus erlotinib.
Methods
Patients were treated with gemcitabine (1000 mg/m2; administered by intravenous infusion on days 1, 8, and 15 every 4 weeks) and erlotinib (given orally at 100 mg/day). We compared the frequencies of HLA alleles in patients who did and did not develop ILD.
Results
A total of 57 patients were treated, and 4 patients (7.0 %) developed ILD. The combination of HLA-B*15:01 and DRB1*15:01 was observed in 2 of 4 patients (50 %) with ILD and in only 1 of 53 patients without ILD (2 %) resulting in odds ratio of 52.0 (95 % CI 3.2–842.5; p = 0.011).
Conclusion
These results suggest that the combination of HLA-B*15:01 and DRB1*15:01 is associated with ILD in Japanese patients with advanced pancreatic cancer receiving gemcitabine plus erlotinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a phase III study, gemcitabine plus erlotinib improved overall survival significantly compared with gemcitabine alone in patients with advanced pancreatic cancer [1]. As single agents, gemcitabine and erlotinib cause interstitial lung disease (ILD), which may be life threatening. A higher incidence of ILD was reported in Japanese patients compared to white patients treated with either erlotinib or gemcitabine. In patients with non-small cell lung cancer, erlotinib treatment resulted in ILD in 4.3 % of Japanese patients and 2.7 % of white patients [2, 3]. Similarly, the incidence of ILD in patients treated with gemcitabine for pancreatic cancer was 2.1 % of Japanese patients and 0.4 % of white patients [1, 4]. In a phase III study of combination chemotherapy with gemcitabine and erlotinib for pancreatic cancer conducted in Canada, the incidence of ILD was 3.5 %, while a higher incidence of 8.5 % was reported in a Japanese phase II study of the same chemotherapy [5]. Although the etiology of gemcitabine plus erlotinib-induced ILD is unclear, the higher incidence of ILD in Japanese cancer patients suggests an interethnic difference.
Risk factors for ILD identified in a post-marketing surveillance study of erlotinib plus gemcitabine (POLARIS) in Japanese pancreatic cancer patients were the number of metastatic sites at >3 [hazard ratio (HR) 4.2 (95 % CI 2.2–8.2)] and concurrent/previous lung diseases [HR 2.2 (95 % CI 1.1–4.5)] [6]. In a phase IV surveillance study of erlotinib (POLARSTAR) as a single agent in Japanese patients with non-small cell lung cancer, concurrent/previous ILD [HR 3.2 (95 % CI 2.4–4.3)], emphysema or chronic obstructive pulmonary disease [HR 1.9 (95 % CI 1.4–2.4)], lung infection [HR 1.6 (95 % CI 1.1–2.2)], smoking history [HR 2.3 (95 % CI 1.7–3.0)], and period from initial cancer diagnosis to the start of the treatment [<360 days; HR 0.6 (95 % CI 0.5–0.7)] were associated with ILD [2]. For gemcitabine, prior thoracic radiotherapy [HR 26.3 (95 % CI 3.4–202.1)] and pre-existing pulmonary fibrosis [HR 6.5 (95 % CI 1.1–38.1)] were identified as significant risk factors for developing ILD in Japanese patients with non-small cell lung cancer and pancreatic cancer [7].
The factors described above do not explain the higher incidence of ILD in Japanese patients treated with gemcitabine, erlotinib, or their combination compared to that of white patients. However, genetic factors may explain the observed interethnic difference in ILD. A genome-wide linkage study identified the mucin (MUC) 5B gene as associated with familial interstitial pneumonia and idiopathic pulmonary fibrosis (IPF) in a white population [8], while an association between the MUC4 gene and ILD induced by epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) or acute exacerbation of IPF was reported in Japanese patients [9]. A few previous studies also reported the association between human leukocyte antigen (HLA) alleles and IPF or drug hypersensitivity [10–16]. However, a relationship between HLA alleles and anticancer drug-induced ILD has not been elucidated. To elucidate genetic backgrounds correlated with a higher incidence of ILD by gemcitabine and erlotinib in Japanese patients, we conducted a prospective study to analyze the association between HLA alleles and ILD in patients with advanced pancreatic cancer receiving gemcitabine plus erlotinib.
Patients and methods
Patients
Patients (20–80 years old) with histological or cytological evidence of unresectable locally advanced or metastatic pancreatic cancer were enrolled. Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, adequate hematologic, renal, and hepatic functions, and a life expectancy of at least 2 months. Patients with a concurrent or previous history of ILD, IPF, pneumoconiosis, drug-induced pneumonia, pulmonary emphysema, or chronic obstructive pulmonary disease were excluded. Patients treated with radiation to the chest or lung resection, as well as gemcitabine or EGFR-TKI within 3 months, were also excluded.
Study design and treatment
Patients were treated with gemcitabine (1000 mg/m2 by intravenous infusion for 30 min on days 1, 8, and 15 every 4 weeks) and erlotinib (given orally at 100 mg/day) [1]. The treatment continued until disease progression, unacceptable toxicities, or refusal by patients. This study was approved by the Institutional Review Boards of Kobe University Hospital, Kobe City Medical Center General Hospital, and National Institute of Health Sciences. All patients provided written informed consent.
Samples and HLA typing
Blood samples were collected from all patients within the 2 weeks before starting the treatment. DNA for HLA typing was extracted from peripheral lymphocytes. HLA-A, B, and DRB1 alleles were determined using the Luminex 200 system (Luminex, Austin, TX, USA) and WAKFlow HLA typing kit (Wakunaga, Hiroshima, Japan). Data were analyzed using WAKFlow typing software (Wakunaga, Hiroshima, Japan) in the HLA Foundation Laboratory (Kyoto, Japan) [17].
Assessments
Chest X-ray was performed weekly for the first 4 weeks and every 2 weeks thereafter. Chest computed tomography (CT) scan was performed every 4 weeks. Antitumor efficacy was evaluated by CT every 8 weeks based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Adverse events were assessed using Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical analysis
Statistical analyses were performed using SPSS software version 21.0. Allele frequencies of HLA in patients with or without ILD were compared using Fisher’s exact test with 2 × 2 tables. P values <0.05 were considered statistically significant.
We considered that at least 4 ILD events were necessary for the association study. In a Japanese phase II study of gemcitabine plus erlotinib for pancreatic cancer, the incidence of ILD was 8.5 %; therefore, the original planned sample size was approximately 50 to detect 4 ILD events.
Results
Between February 2013 and July 2015, 57 patients were enrolled from two institutions: Kobe University Hospital (n = 44) and Kobe City Medical Center General Hospital (n = 13). Of these patients, 4 patients (7.0 %) developed ILD. Baseline characteristics are shown in Table 1. Median age and smoking history were similar between the two groups.
The median duration of the treatment was 1.7 months (range 1.0–3.3) in patients who developed ILD and 2.7 (0.1–16.0) months in those without ILD. The most common reason for discontinuation was disease progression (45 patients, 78.9 %) evaluated by RECIST criteria. Treatment-related adverse events lead to treatment discontinuation in 7 patients (12.3 %) due to malaise, diarrhea, dysgeusia, and ILD (4 patients, 7.0 %). Treatment was discontinued due to adverse events not related to the treatment in 4 patients (7.0 %). One patient (1.8 %) stopped the treatment due to progressive symptoms of the primary disease.
Median progression-free survival was 2.8 (1.0–3.5) months in patients with ILD and 2.8 (0.1–16.8) months in patients without ILD. Partial responses were achieved in 2 patients (3.5 %), and stable disease (SD) was observed in 27 patients (47.4 %). Best response in four patients who developed ILD was SD in 1 and disease progression in 3 patients.
Chest CT scans of 4 patients who developed ILD at the onset of ILD are shown in Fig. 1. ILD developed at 4, 6, 8, and 12 weeks after the start of the treatment in the 4 patients, respectively. ILD was asymptomatic in 1 patient (grade 1), while it was associated with mild symptoms including productive cough, dyspnea, or fever in 3 patients (grade 2). ILD was improved by treatment discontinuation in all patients.
a Chest CT scans of a 65-year-old female non-smoker with lung and liver metastases at 8 weeks. b Chest CT scans of a 64-year-old female non-smoker with locally advanced cancer at 12 weeks. c Chest CT scans of a 76-year-old male ex-smoker with lung metastasis at 4 weeks. d Chest CT scans of a 70-year-old male ex-smoker with liver metastasis at 6 weeks
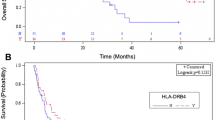
In HLA analysis, 2 of 4 patients who developed ILD harbored HLA-B*15:01, B*40:06, DRB1*09:01, and DRB1*15:01, but their association with ILD was not statistically significant. The frequencies of HLA alleles in patients with or without ILD are listed in the Supplementary Table. However, the combination of HLA-B*15:01 and DRB1*15:01 was observed in 2 of the 4 patients (50 %) who developed ILD, while only 1 of 53 patients (2 %) who did not develop ILD harbored this combination, resulting in an odds ratio (OR) of 52.0 (95 % CI 3.2–842.5; p = 0.01, Table 2).
Discussion
Our results demonstrate that the combination of HLA-B*15:01 and DRB1*15:01 is over-represented significantly in Japanese patients with advanced pancreatic cancer who developed ILD after the treatment with gemcitabine plus erlotinib. Although the number of patients was small, the OR of 52.0 was much higher than the OR (2.2–4.2) of previously reported risk factors for ILD caused by gemcitabine and erlotinib [6].
An over-representation of HLA-DRB1*15:01 was reported in white patients with IPF not associated with drug therapy [10]. IPF is a clinical form of ILD and the most common type of ILD. Other HLA types (HLA-A*3, B*14, B*15, or B*40, and combination of A2B15, A2B40, A11B15, A24B58, or A30B40) were also associated with IPF in a Han Chinese population [11]. Therefore, drug-induced ILD may be associated with certain types of HLA.
HLA alleles are associated with drug hypersensitivity. Carbamazepine-induced Stevens–Johnson syndrome is associated with HLA-B*15:02 in Chinese patients and HLA-A*31:01 in Japanese or European patients [12–14]. HLA-B*58:01 and HLA-B*57:01 are over-represented in allopurinol-induced severe cutaneous reactions [15] and abacavir hypersensitivity [16], respectively. Among anticancer drugs, hepatotoxicity by lapatinib is associated with HLA-DQA1*02:01 or DRB1*07:01 [18]. The frequencies of these alleles are 10–15 % in white patients and 0.3–0.8 % in Japanese patients [17, 19].
With regard to drug-induced ILD, HLA-A*31:01 and HLA-DRB1*15:02 are known to be associated with methotrexate-induced ILD in Japanese patients with rheumatoid arthritis (RA), which induce ILD as a complication [20, 21], and DRB1*15 and *16 were associated with a risk of ILD in Japanese RA patients regardless of methotrexate treatment [22].
These observations suggest that HLA plays an important role in drug-induced ILD, and our findings demonstrate a significant association of certain HLA types with drug-induced ILD. The mechanism of the association between HLA and ILD is still unclear, although an in vitro study demonstrated that a direct interaction between HLA and carbamazepine activates T cells [23]. Drugs or their metabolites may act as haptens and non-covalently bind to the HLA molecule [24].
The incidence of ILD by gemcitabine plus erlotinib for advanced pancreatic cancer was 8.5 % in a Japanese phase II study, 6.2 % in a post-marketing surveillance study, and 7.0 % in our study. We carefully excluded patients with risk factors for ILD from our study. Nonetheless, a significant number of patients developed ILD. These incidences are obviously higher than those reported in white patients. In our prospective study, an association between drug-induced ILD and HLA-B*15:01/DRB1*15:01 was observed. Differences in the population frequency of the HLA alleles may explain, at least in part, the interethnic differences in the incidence of ILD induced by gemcitabine and erlotinib.
In conclusion, these results suggest that the combination of HLA-B*15:01 and DRB1*15:01 is associated with ILD in Japanese patients with advanced pancreatic cancer receiving gemcitabine plus erlotinib.
References
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials G (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966. doi:10.1200/JCO.2006.07.9525
Gemma A, Kudoh S, Ando M, Ohe Y, Nakagawa K, Johkoh T, Yamazaki N, Arakawa H, Inoue Y, Ebina M, Kusumoto M, Kuwano K, Sakai F, Taniguchi H, Fukuda Y, Seki A, Ishii T, Fukuoka M (2014) Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non-small-cell lung cancer. Cancer Sci 105(12):1584–1590. doi:10.1111/cas.12550
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L, National Cancer Institute of Canada Clinical Trials G (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353(2):123–132. doi:10.1056/NEJMoa050753
Tamiya A, Endo M, Shukuya T, Igawa S, Tsuya A, Nakamura Y, Murakami H, Takahashi T, Boku N, Yamamoto N (2009) Features of gemcitabine-related severe pulmonary toxicity: patients with pancreatic or biliary tract cancer. Pancreas 38(7):838–840. doi:10.1097/MPA.0b013e3181ad97cf
Okusaka T, Furuse J, Funakoshi A, Ioka T, Yamao K, Ohkawa S, Boku N, Komatsu Y, Nakamori S, Iguchi H, Ito T, Nakagawa K, Nakachi K (2011) Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci 102(2):425–431. doi:10.1111/j.1349-7006.2010.01810.x
Furuse J, Gemma A, Hatori T (2014) Final safety analysis of erlotinib plus gemcitabine in a post-marketing surveillance study (POLARIS) of >800 Japanese pancreatic cancer patients. Ann Oncol 25(Suppl 4):iv210–iv253. doi:10.1093/annonc/mdu334.89
Umemura S, Yamane H, Suwaki T, Katoh T, Yano T, Shiote Y, Takigawa N, Kiura K, Kamei H (2011) Interstitial lung disease associated with gemcitabine treatment in patients with non-small-cell lung cancer and pancreatic cancer. J Cancer Res Clin Oncol 137(10):1469–1475. doi:10.1007/s00432-011-1013-1
Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA (2011) A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364(16):1503–1512. doi:10.1056/NEJMoa1013660
Siihara J IK, Miyazawa H et al (2014) The 55 th annual meeting of the Japan lung cancer society
Xue J, Gochuico BR, Alawad AS, Feghali-Bostwick CA, Noth I, Nathan SD, Rosen GD, Rosas IO, Dacic S, Ocak I, Fuhrman CR, Cuenco KT, Smith MA, Jacobs SS, Zeevi A, Morel PA, Pilewski JM, Valentine VG, Gibson KF, Kaminski N, Sciurba FC, Zhang Y, Duncan SR (2011) The HLA class II Allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One 6(2):e14715. doi:10.1371/journal.pone.0014715
Zhang J, Xu DJ, Xu KF, Wu B, Zheng MF, Chen JY, Huang JA (2012) HLA-A and HLA-B gene polymorphism and idiopathic pulmonary fibrosis in a Han Chinese population. Respir Med 106(10):1456–1462. doi:10.1016/j.rmed.2012.06.015
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT (2004) Medical genetics: a marker for Stevens–Johnson syndrome. Nature 428(6982):486. doi:10.1038/428486a
Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, Ikezawa Z, Iijima M, Shiohara T, Hashimoto K, Kamatani N, Nakamura Y (2011) Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet 20(5):1034–1041. doi:10.1093/hmg/ddq537
McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D, Carrington M, Sills GJ, Marson T, Jia X, de Bakker PI, Chinthapalli K, Molokhia M, Johnson MR, O’Connor GD, Chaila E, Alhusaini S, Shianna KV, Radtke RA, Heinzen EL, Walley N, Pandolfo M, Pichler W, Park BK, Depondt C, Sisodiya SM, Goldstein DB, Deloukas P, Delanty N, Cavalleri GL, Pirmohamed M (2011) HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med 364(12):1134–1143. doi:10.1056/NEJMoa1013297
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, Chen MJ, Lai PC, Wu MS, Chu CY, Wang KH, Chen CH, Fann CS, Wu JY, Chen YT (2005) HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 102(11):4134–4139. doi:10.1073/pnas.0409500102
Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, Fling ME, Stocum M, Bowman C, Thurmond LM, Roses AD (2002) Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 359(9312):1121–1122. doi:10.1016/S0140-6736(02)08158-8
Ikeda N, Kojima H, Nishikawa M, Hayashi K, Futagami T, Tsujino T, Kusunoki Y, Fujii N, Suegami S, Miyazaki Y, Middleton D, Tanaka H, Saji H (2015) Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens 85(4):252–259. doi:10.1111/tan.12536
Spraggs CF, Schaid DJ, Parham LR, McDonnell SK, Briley LP, King KS, Rappold E, Goss PE (2012) Abstract PD 10-05: HLA-DQA1*02:01/DRB1*07:01 as a biomarker for lapatinib-induced hepatotoxicity: prospective confirmation in a large randomised clinical trial (TEACH, EGF105485). Cancer Res 72(Suppl 24). doi:10.1158/0008-5472.SABCS12-PD10-05
The Allele Frequency. (http://www.allelefrequencoes.net/)
Furukawa H, Oka S, Shimada K, Rheumatoid Arthritis-Interstitial Lung Disease Study C, Tsuchiya N, Tohma S (2013) HLA-A*31:01 and methotrexate-induced interstitial lung disease in Japanese rheumatoid arthritis patients: a multidrug hypersensitivity marker? Ann Rheum Dis 72(1):153–155. doi:10.1136/annrheumdis-2012-201944
Mori S, Koga Y, Sugimoto M (2012) Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med 106(11):1591–1599. doi:10.1016/j.rmed.2012.07.006
Furukawa H, Oka S, Shimada K, Sugii S, Ohashi J, Matsui T, Ikenaka T, Nakayama H, Hashimoto A, Takaoka H, Arinuma Y, Okazaki Y, Futami H, Komiya A, Fukui N, Nakamura T, Migita K, Suda A, Nagaoka S, Tsuchiya N, Tohma S (2012) Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PLoS One 7(5):e33133. doi:10.1371/journal.pone.0033133
Wei CY, Chung WH, Huang HW, Chen YT, Hung SI (2012) Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens–Johnson syndrome. J Allergy Clin Immunol 129(6):1562.e5–1569.e5. doi:10.1016/j.jaci.2011.12.990
Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, Miles JJ, Kjer-Nielsen L, Gras S, Williamson NA, Burrows SR, Purcell AW, Rossjohn J, McCluskey J (2012) Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 486(7404):554–558. doi:10.1038/nature11147
Acknowledgments
We thank all the participants in this study, including patients, caregivers, physicians, and medical workers.
Funding
This work was supported in part by research funding by Kobe University and by Research on Regulatory Harmonization and Evaluation of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics from Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nishimura, M., Toyoda, M., Takenaka, K. et al. The combination of HLA-B*15:01 and DRB1*15:01 is associated with gemcitabine plus erlotinib-induced interstitial lung disease in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 77, 1165–1170 (2016). https://doi.org/10.1007/s00280-016-3026-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3026-6