Abstract
Background
Bortezomib is a selective reversible proteasome inhibitor with proapoptotic effects. Preclinical and phase I clinical data suggest activity of bortezomib in NSCLC, either as monotherapy or in combination with chemotherapeutic agents including gemcitabine and cisplatin.
Methods
Chemotherapy-naïve patients with inoperable stage IIIB or IV NSCLC were administered bortezomib 1 mg/m2 i.v. on days 1 and 8, and starting on day 21 (cycle 2), bortezomib (days 1 and 8) in combination with gemcitabine 1000 mg/m2, (days 1 and 8), and cisplatin 70 mg/m2 (day 1) in cycles of 21 days. Up to 8 cycles of combination therapy could be administered; single-agent bortezomib was continued until disease progression or unacceptable toxicity.
Results
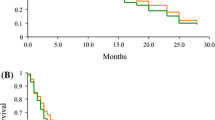
Fifty-three patients [median age 66 years; 79.2 % male; 96.2 % stage IV; performance status (ECOG) 0/1 73.6/26.4 %; adenocarcinoma 45.3 %, squamous cell carcinoma 41.5 %] were enrolled. All patients were evaluable for toxicity and 43 for efficacy. Grade 3–4 hematologic toxicity consisted of neutropenia (22.6 %) and thrombocytopenia (17 %). Grade 2–4 non-hematologic adverse events were fever (9.4 %), fatigue (20.8 %), infection (18.9 %), and dyspnea (15.1 %). There was no >grade 2 neurotoxicity. Febrile neutropenia occurred in two (1.9 %) patients, and there were three possibly treatment-related deaths (5.4 %). In the intention-to-treat population, the objective response rate was 17 % (95 % CI 6.9–27.1 %). No difference in response rate was observed for squamous versus other histology (18.2 vs. 16.1 %, p = 0.845). The median progression-free survival was 2.5 months, the median overall survival 10.6 months and the 1-year survival rate 38.1 %.
Conclusion
The incorporation of bortezomib into the gemcitabine/cisplatin regimen, in the dose and schedule used in this study, could not improve the efficacy of the chemotherapy regimen and has not to be further investigated.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249. doi:10.3322/caac.20006
Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50(1):7–33
Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR (2004) American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 22(2):330–353
Azzoli CG, Temin S, Aliff T, Baker S Jr, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pao W, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G (2011) 2011 focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 29(28):3825–3831. doi:10.1200/JCO.2010.34.2774
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 355(24):2542–2550. doi:10.1056/NEJMoa061884
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27(8):1227–1234. doi:10.1200/JCO.2007.14.5466
Mack PC, Davies AM, Lara PN, Gumerlock PH, Gandara DR (2003) Integration of the proteasome inhibitor PS-341 (Velcade) into the therapeutic approach to lung cancer. Lung Cancer 41(Suppl 1):S89–S96. doi:10.1016/S0169-5002(03)00149-1
Schenkein DP (2004) Use of proteasome inhibition in the treatment of lung cancer. Clin Lung Cancer 6(Suppl 2):S89–S96
Yang Y, Ikezoe T, Saito T, Kobayashi M, Koeffler HP, Taguchi H (2004) Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci 95(2):176–180
Denlinger CE, Rundall BK, Keller MD, Jones DR (2004) Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann Thorac Surg 78(4):1207–1214. doi:10.1016/j.athoracsur.2004.04.029
Mortenson MM, Schlieman MG, Virudachalam S, Bold RJ (2004) Effects of the proteasome inhibitor bortezomib alone and in combination with chemotherapy in the A549 non-small-cell lung cancer cell line. Cancer Chemother Pharmacol 54(4):343–353. doi:10.1007/s00280-004-0811-4
Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, Canales C, Daud A, Spriggs DR (2002) A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 8(8):2505–2511
Fanucchi MP, Fossella FV, Belt R, Natale R, Fidias P, Carbone DP, Govindan R, Raez LE, Robert F, Ribeiro M, Akerley W, Kelly K, Limentani SA, Crawford J, Reimers HJ, Axelrod R, Kashala O, Sheng S, Schiller JH (2006) Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol 24(31):5025–5033. doi:10.1200/JCO.2006.06.1853
Johnson S, Algazy K, Miller D et al (2005) Phase 2 clinical/pharmacodynamic trial of the proteasome inhibitor PS-341 in advanced NSCLC. Proc Am Soc Clin Oncol; Chicago, Abstr 810
Voortman J, Smit EF, Kuenen BC, Peters GJ, van de Velde H, Giaccone G (2005) A phase 1B, open-label, dose-escalation study of bortezomib in combination with gemcitabine and cisplatin in the first-line treatment of patients with advanced solid tumors. ECCO 2005, abstr 1468
Voortman J, Smit EF, Honeywell R, Kuenen BC, Peters GJ, van de Velde H, Giaccone G (2007) A parallel dose-escalation study of weekly and twice-weekly bortezomib in combination with gemcitabine and cisplatin in the first-line treatment of patients with advanced solid tumors. Clin Cancer Res 13(12):3642–3651. doi:10.1158/1078-0432.CCR-07-0061
Davies AM, Chansky K, Lara PN Jr, Gumerlock PH, Crowley J, Albain KS, Vogel SJ, Gandara DR (2009) Bortezomib plus gemcitabine/carboplatin as first-line treatment of advanced non-small cell lung cancer: a phase II Southwest Oncology Group Study (S0339). J Thorac Oncol 4(1):87–92. doi:10.1097/JTO.0b013e3181915052
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92(3):205–216
National Cancer Institute (2006) Common Terminology Criteria for Adverse Events (CTCAE). Cancer Therapy Evaluation Program. Common toxicity criteria. Version 3.0. DCTD, NCI, NIH, DHHS edn
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Collet D (1994) Modelling survival data in medical research, 3rd edn. Blackwell Scientific, Oxford
Cox DR (1970) The analysis of binary data, 1st edn. Methuen, London
Crino L, Scagliotti GV, Ricci S, De Marinis F, Rinaldi M, Gridelli C, Ceribelli A, Bianco R, Marangolo M, Di Costanzo F, Sassi M, Barni S, Ravaioli A, Adamo V, Portalone L, Cruciani G, Masotti A, Ferrara G, Gozzelino F, Tonato M (1999) Gemcitabine and cisplatin versus mitomycin, ifosfamide, and cisplatin in advanced non-small-cell lung cancer: a randomized phase III study of the Italian Lung Cancer Project. J Clin Oncol 17(11):3522–3530
Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U, Mattson K, Manegold C, Palmer MC, Gregor A, Nguyen B, Niyikiza C, Einhorn LH (2000) Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 18(1):122–130
Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G, Altavilla G, Adamo V, Ceribelli A, Clerici M, Di Costanzo F, Frontini L, Tonato M (2002) Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 20(21):4285–4291
Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, Smit H, Gaafar R, Biesma B, Manegold C, Neymark N, Giaccone G (2003) Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group—EORTC 08975. J Clin Oncol 21(21):3909–3917
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26(21):3543–3551. doi:10.1200/JCO.2007.15.0375
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352(24):2487–2498. doi:10.1056/NEJMoa043445
Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, Jaye DL, Francis D, Giusti S, Torre C, Barlogie B, Berenson JR, Singhal S, Schenkein DP, Esseltine DL, Anderson J, Xiao H, Heffner LT, Anderson KC (2005) Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood 106(12):3777–3784. doi:10.1182/blood-2005-03-1173
Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A, Guglielmelli T, Benevolo G, Callea V, Rizzo V, Cangialosi C, Musto P, De Rosa L, Liberati AM, Grasso M, Falcone AP, Evangelista A, Cavo M, Gaidano G, Boccadoro M, Palumbo A (2010) Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood 116(23):4745–4753. doi:10.1182/blood-2010-07-294983
Acknowledgments
This work was supported by investigational Grants from the Cretan Association for Biomedical Research (CABR) and Janssen Pharmaceuticals. G. F. and A. M. were recipients of a CABR clinical fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EK has received an honorarium from Janssen-Cilag Pharmaceutical S. A. C. I. All the other authors disclosed no potential conflicts of interest.
Additional information
On behalf of the Lung Cancer Working Group of the Hellenic Oncology Research Group (HORG).
ClinicalTrilas.gov Identifier: NCT01633645.
Rights and permissions
About this article
Cite this article
Kontopodis, E., Kotsakis, A., Kentepozidis, N. et al. A phase II, open-label trial of bortezomib (VELCADE®) in combination with gemcitabine and cisplatin in patients with locally advanced or metastatic non-small cell lung cancer. Cancer Chemother Pharmacol 77, 949–956 (2016). https://doi.org/10.1007/s00280-016-2997-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2997-7