Abstract
Purpose
No standard salvage chemotherapy has been identified for metastatic pancreatic adenocarcinoma (mPA), and there is an urgent need for active agents against this disease. This phase II trial explored the activity of trabectedin in mPA progressing after gemcitabine-based first-line chemotherapy.
Methods
Patients with gemcitabine-resistant disease received trabectedin 1.3 mg/m2 as a 3-h intravenous continuous infusion every 3 weeks until disease progression or unacceptable toxicity or for a maximum of 6 months. The primary endpoint was progression-free survival rate at 6 months (PFS-6). Since trabectedin modulates the production of selected inflammatory mediators, this study also aimed to identify inflammatory biomarkers predictive for response to trabectedin.
Results
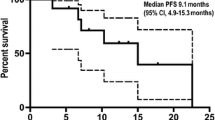
Between February 2011 and February 2012, 25 patients received trabectedin. PFS-6 was 4 %, median PFS 1.9 months (range 0.8–7.4), and median overall survival 5.2 months (range 1.1–24.3). Grade >2 toxicity consisted of neutropenia in 44 % of patients, febrile neutropenia and thrombocytopenia both in 12 %, anemia in 8 %, fatigue in 12 %, and AST and ALT increase in 8 and 4 %, respectively. Trabectedin was shown to modulate the production of inflammatory mediators, and at disease progression, levels of a subgroup of cytokines/chemokines were modified. Furthermore, tissue analysis identified 30 genes associated with better prognosis.
Conclusions
Although it has shown some ability to modulate inflammatory process, single-agent trabectedin had no activity as salvage therapy for mPA.



Similar content being viewed by others
References
Reni M, Cordio S, Milandri C et al (2005) Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: a randomized controlled multicenter phase III trial. Lancet Oncol 6:369–376
Conroy T, Desseigne F, Ychou M et al: Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J 69:1691–1703
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31:1640–1648
Burris HA III, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Pelzer U, Schwaner I, Stieler J et al (2011) Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 47:1676–1681
Reni M, Berardi R, Mambrini A et al (2008) A multi-centre retrospective review of second-line therapy in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 62:673–678
Boeck S, Weigang-Köhler K, Fuchs M et al (2007) Second-line chemotherapy with pemetrexed after gemcitabine failure in patients with advanced pancreatic cancer: a multicenter phase II trial. Ann Oncol 18:745–751
Boeck S, Wilkowski R, Bruns CJ et al (2007) Oral capecitabine in gemcitabine-pretreated patients with advanced pancreatic cancer. Oncology 73:221–227
Cereda S, Rognone A, Mazza E et al (2009) Weekly Epirubicin as salvage therapy in patients with gemcitabine-resistant pancreatic cancer. J Chemother 21:698–700
Ulrich-Pur H, Raderer M, Verena Kornek G et al (2003) Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced adenocarcinoma. Br J Cancer 88:1180–1184
Cereda S, Reni M (2008) Weekly docetaxel as salvage therapy in patients with gemcitabine-refractory metastatic pancreatic cancer. J Chemother 20:509–512
Yi SY, Park YS, Kim HS et al (2009) Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother Pharmacol 63:1141–1145
Ducreux M, Mitry E, Ould-Kaci M et al (2004) Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol 15:467–473
Cereda S, Reni M, Rognone A et al (2011) Salvage therapy with mitomycin and ifosfamide (MI) in patients with gemcitabine-resistant metastatic pancreatic cancer. Chemotherapy 57:156–161
Demols A, Peeters M, Polus M et al (2006) Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 94:481–485
Cantore M, Rabbi C, Fiorentini G et al (2004) Combined irinotecan and oxalipatin in patients with advanced pre-treated pancreatic cancer. Oncology 67:93–97
Reni M, Cereda S, Mazza E et al (2008) PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) regimen as second-line therapy in patients with progressive or recurrent pancreatic cancer after gemcitabine-containing chemotherapy. Am J Clin Oncol 31:145–150
Reni M, Pasetto L, Aprile G et al (2006) Raltitrexed-eloxatin salvage chemotherapy in gemcitabine resistant metastatic pancreatic cancer. Br J Cancer 94:785–791
Yoo C, Hwang JY, Kim JE et al (2009) A randomised phase II study of modified FOLFIRI.3 vs. modified FOLFOX as second-line therapy in patients with gemcitabine refractory advanced pancreatic cancer. Br J Cancer 101:1658–1663
Cereda S, Reni M, Rognone A et al (2010) XELIRI or FOLFIRI as salvage therapy in advanced pancreatic cancer. Anticancer Res 30:4785–4790
D’Incalci M, Galmarini CM (2010) A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther 9:2157–2163
Germano G, Frapolli R, Belgiovine C et al (2013) Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23:249–262
Forouzeh B, Hidalgo M, Denis L et al (2001) Phase I and pharmacokinetic study of the marine derived DNA minor groove binder ET-743 on a weekly × 3 every-4-week schedule in patients with advanced solid malignancies. Proc Am Soc Clin Oncol 20:94a (abstract 373)
Ryan DP, Supko JG, Eder JP et al (2001) Phase I and pharmacokinetic study of ecteinascidin 743 administered as a 72-hour continuous intravenous infusion in patients with solid malignancies. Clin Cancer Res 7:231–242
Taamma A, Misset JL, Riofrio M et al (2001) Phase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-hour continuous infusion in patients with solid tumors. J Clin Oncol 19:1256–1265
Twelves C, Hoekman K, Bowman A et al (2003) Phase I and pharmacokinetic study of Yondelis (Ecteinascidin-743; ET-743) administered as an infusion over 1 h or 3 h every 21 days in patients with solid tumours. Eur J Cancer 39:1842–1851
Del Campo JM, Roszak A et al (2009) Phase II randomized study of trabectedin given as two different every 3 weeks dose schedules (1.5 mg/m2 24 h or 1.3 mg/m2 3 h) to patients with relapsed, platinum-sensitive, advanced ovarian cancer. Ann Oncol 20:1794–1802
Allavena P, Signorelli M, Chieppa M et al (2005) Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res 65:2964–2971
Germano G, Frapolli R, Simone M et al (2010) Antitumor and anti inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res 70:2235–2244
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Zangarini M, Ceriani L, Sala F et al (2014) Quantification of trabectedin in human plasma: validation of a high-performance liquid chromatography-mass spectrometry method and its application in a clinical pharmacokinetic study. Pharm Biomed Anal 95:107–112
Janku F, Tsimberidou A, Garrido-Laguna I et al (2010) PIK3CA, KRAS, and BRAF mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Proc Am Soc Clin Oncol 28:2583 (abstract 2583)
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404–416
Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff IH (1990) Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 8:147–159
Reni M, Cereda S, Balzano P et al (2009) Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 12:2630–2639
Hosein PJ, de Lima Lopes G, Pastorini VH et al (2013) A phase II trial of nab-paclitaxel (NP) as second line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol 36:151–156
Ko AH, Tempero MA, Shan YS et al (2013) A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. J Cancer 4:920–925
Del Campo JM, Sessa CD, Krasner CN et al (2013) Trabectedin as single agent in relapsed advanced ovarian cancer: results from a retrospective pooled analysis of three phase II trials. Med Oncol 30:435–445
Acknowledgments
This work was supported by unrestricted grant from PharmaMar which also supplied trabectedin. PharmaMar did not have any role in study design, collection, analysis, interpretation of data, or writing the report. We thank Ray Hill, an independent medical writer, who provided medical writing support and journal styling prior to submission on behalf of Health Publishing and Services Srl.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Belli, C., Piemonti, L., D’Incalci, M. et al. Phase II trial of salvage therapy with trabectedin in metastatic pancreatic adenocarcinoma. Cancer Chemother Pharmacol 77, 477–484 (2016). https://doi.org/10.1007/s00280-015-2932-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2932-3