Abstract
Purpose
To characterize cobimetinib pharmacokinetics and evaluate impact of clinically relevant covariates on cobimetinib pharmacokinetics.
Methods
Plasma samples (N = 4886) were collected from 487 patients with various solid tumors (mainly melanoma) in three clinical studies (MEK4592g, NO25395, GO28141). Cobimetinib was administered orally, once daily on either a 21-day-on/7-day-off, 14-day-on/14-day-off or 28-day-on schedule in a 28-day dosing cycle as single agent or in combination with vemurafenib. Cobimetinib doses ranged from 2.1 to 125 mg. NONMEM was used for pharmacokinetic analysis.
Results
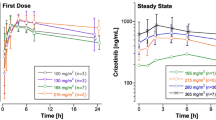
A linear two-compartment model with first-order absorption, lag time and first-order elimination described cobimetinib pharmacokinetics. The typical estimates (inter-individual variability) of apparent clearance (CL/F), central volume of distribution (V2/F) and terminal half-life were 322 L/day (58 %), 511 L (49 %) and 2.2 days, respectively. Inter-occasion variability on relative bioavailability was estimated at 46 %. CL/F decreased with age. V2/F increased with body weight (BWT). However, the impact of age and BWT on cobimetinib steady-state exposure (peak and trough concentrations and AUC following the recommended daily dose of 60 mg 21-day-on/7-day-off) was limited (<25 % changes across the distribution of age and BWT). No significant difference in cobimetinib pharmacokinetics or steady-state exposure was observed between patient subgroups based on sex, renal function, ECOG score, hepatic function tests, race, region, cancer type, and co-administration of moderate and weak CYP3A inducers or inhibitors and vemurafenib.
Conclusion
A population pharmacokinetic model was developed for cobimetinib in cancer patients. Covariates had minimal impact on steady-state exposure, suggesting no need for dose adjustments and supporting the recommended dose for all patients.



Similar content being viewed by others
References
Hoeflich KP, Merchant M, Orr C et al (2012) Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res 72:210–219
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Sovak MA, Chang I, Choong N, Hack SP, McArthur GA, Ribas A (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371(20):1867–1876
Musib L, Choo E, Deng Y, Eppler S, Rooney I, Chan IT, Dresser MJ (2013) Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm 10(11):4046–4054
Deng Y, Musib L, Choo E, Chapple M, Burke S, Johnson J, Eppler S, Dean B (2014) Determination of cobimetinib in human plasma using protein precipitation extraction and high-performance liquid chromatography coupled to mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 1(972):117–123
Musib L, Eppler S, Choo E, Deng A, Miles D, Hsu B, Rosen L, Sikic B, LoRusso P, Ma W, Goldman J, Fisher G, Weise A, Dy G, Chan I, Ware J (2011) Clinical pharmacokinetics of GDC-0973, an oral MEK inhibitor, in cancer patients: data from a phase 1 study. Presented at American Association for Cancer Research, 102nd annual meeting; April 2–6, 2011; Orlando, FL
Ribas A, Gonzalez R, Pavlick A, Hamid O, Gajewski TF, Daud A, Flaherty L, Logan T, Chmielowski B, Lewis K, Kee D, Boasberg P, Yin M, Chan I, Musib L, Choong N, Puzanov I, McArthur GA (2014) Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol 15(9):954–965
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20
Guidance for Industry Population Pharmacokinetics, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Feb 1999. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072137.pdf
Committee for Medicinal Products for Human Use (CHMP), Guideline on reporting the results of population pharmacokinetic analyses, Doc. Ref. CHMP/EWP/185990/06, London, 21 June 2007. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003067.pdf
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151
Ette EI (1997) Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37:486–495
Savic RM, Karlsson MO (2009) Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569
US Food and Drug Administration (2010) guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. US Food and Drug administration, Rockville. http://www.fda.gov/downloads/Drugs/guidanceComplianceregulatoryInformation/guidances/UCM204959.pdf
Choo E, Takahashi R, Rooney I, Gates M, Deng Y, Musib L (2013) Assessing human absorption, metabolism, routes of excretion and the contribution of intestinal metabolism to the oral clearance of cobimetinib, a MEK inhibitor. Mol Cancer Ther 12:B160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Kelong Han, Jin Jin, Stephen Eppler, Nicholas Choong, Stephen P Hack, Nalin Tikoo, Mark Dresser, Luna Musib and Nageshwar Budha receive salary from Genentech and hold stocks of Roche Pharmaceuticals; in addition, Jin Jin and Luna Musib also hold stock in Eli Lilly; Mathilde Marchand and Rene Bruno receive salary from Pharsight Consulting Services.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Correlation between cobimetinib pharmacokinetic parameters from the base model and age and body weight. Blue lines represent the locally weighted scatterplot smoothing (LOESS). CL/F = apparent clearance (L/day); V2/F = apparent central volume of distribution (L). (PDF 116 kb)
Supplementary Fig. 2
Correlation between cobimetinib apparent clearance CL/F (L/day) from the final model and clinically relevant covariates that were not statistically significant. (PDF 179 kb)
Supplementary Fig. 3
Correlation between cobimetinib apparent central volume of distribution V2/F (L) from the final model and clinically relevant covariates that were not statistically significant. (PDF 173 kb)
Supplementary Fig. 4
Goodness-of-fit plots for the final PK model of cobimetinib. Gray lines: LOESS (locally weighted scatterplot smoothing; some of the smooth lines were constrained to rich data). (PDF 1357 kb)
Rights and permissions
About this article
Cite this article
Han, K., Jin, J.Y., Marchand, M. et al. Population pharmacokinetics and dosing implications for cobimetinib in patients with solid tumors. Cancer Chemother Pharmacol 76, 917–924 (2015). https://doi.org/10.1007/s00280-015-2862-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2862-0