Abstract
Purpose
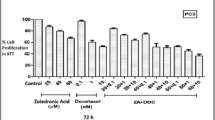
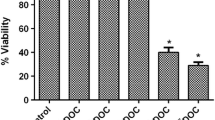
Docetaxel (DTX) is widely used for the treatment of metastatic prostate and breast cancers. Despite the clinical success of DTX, drug-related cumulative toxicity restricts its clinical use in cancer therapy. Thus, there is an urgent need for new therapeutic options. Octreotide (OCT) is a synthetic somatostatin analog that induces apoptosis in different cancer cell lines in vitro. In this study, we investigated the possible synergistic apoptotic effects of DTX in combination with OCT in prostate and breast cancer cell lines.
Methods
The XTT cell viability assay was used to determine cytotoxicity. Apoptosis was evaluated by Cell Death Detection ELISAPlus Kit. The expression levels of apoptotic proteins were assessed by human apoptosis antibody array. Levels of SSTR2 and SSTR5 proteins were determined by western blot analysis.
Results
DTX and OCT combination induced apoptosis in both breast and prostate cancer cells in a concentration- and time-dependent manner. Moreover, combination treatment resulted in inhibition of anti-apoptotic proteins such as Bcl-2 and Bcl-xL and induction of pro-apoptotic proteins Bax, Cytochrome c and IAPs in all of the tested cancer cell lines. SSTR2 and SSTR5 protein levels were induced as compared to any agent alone.
Conclusions
These results indicate that this combination treatment is a significant inducer of apoptosis in a synergistic manner in breast and prostate cancer cells. This strong synergism helps to lower the dose of DTX in both types of cancers, thus letting DTX to be used for longer periods by delaying resistance development and lesser side effects.



Similar content being viewed by others
References
van Oosterom AT, Schrijvers D, Schriivers D (1995) Docetaxel (Taxotere), a review of preclinical and clinical experience. Part II: Clinical experience. Anticancer Drugs 6:356–368
Hwang C (2012) Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol 6:329–340
Madan RA, Pal SK, Sartor O, Dahut WL (2011) Overcoming chemotherapy resistance in prostate cancer. Clin Cancer Res 17:3892–3902
Galsky MD, Small AC, Tsao CK, Oh WK (2012) Clinical development of novel therapeutics for castration-resistant prostate cancer: historic challenges and recent successes. CA Cancer J Clin 62:299–308
Sartor O, Michels RM, Massard C, de Bono JS (2011) Novel therapeutic strategies for metastatic prostate cancer in the post-docetaxel setting. Oncologist 16:1487–1497
Bijnsdorp IV, Kruyt FA, Gokoel S, Fukushima M, Peters GJ (2008) Synergistic interaction between trifluorothymidine and docetaxel is sequence dependent. Cancer Sci 11:2302–2308
Karabulut B, Erten C, Gul MK, Cengiz E, Karaca B, Kucukzeybek Y, Gorumlu G, Atmaca H, Uzunoglu S, Sanli UA, Baran Y, Uslu R (2009) Docetaxel/zoledronic acid combination triggers apoptosis synergistically through downregulating antiapoptotic Bcl-2 protein level in hormone-refractory prostate cancer cells. Cell Biol Int 2:239–246
Mettinen S, Grenman S, Ylikomi T (2009) Inhibition of P-glycoprotein-mediated docetaxel efflux sensitizes ovarian cancer cells to concomitant docetaxel and SN-38 exposure. Anticancer Drugs 4:267–276
He Y, Yuan XM, Lei P, Wu S, Xing W, Lan XL, Zhu HF, Huang T, Wang GB, An R, Zhang YX, Shen GX (2009) The antiproliferative effects of somatostatin receptor subtype 2 in breast cancer cells. Acta Pharmacol Sin 7:1053–1059
Hejna M, Schmidinger M, Raderer M (2002) The clinical role of somatostatin analogues as antineoplastic agents: much ado about nothing? Ann Oncol 5:653–668
Bruns C, Raulf F, Hoyer D, Schloos J, Lübbert H, Weckbecker G (1996) Binding properties of somatostatin receptor subtypes. Metabolism 45:17–20
Follet J, Corcos L, Baffet G, Ezan F, Morel F, Simon B, Le Jossic-Corcos C (2012) The association of statins and taxanes: an efficient combination trigger of cancer cell apoptosis. Br J Cancer 4:685–692
Feleszko W, Zagozdzon R, Golab J, Jakobisiak M (1998) Potentiated antitumour effects of cisplatin and lovastatin against MmB16 melanoma in mice. Eur J Cancer 3:406–411
Agarwal B, Bhendwal S, Halmos B, Moss SF, Ramey WG, Holt PR (1999) Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res 8:2223–2229
Wang W, Collie-Duguid E, Cassidy J (2002) Cerivastatin enhances the cytotoxicity of 5-fluorouracil on chemosensitive and resistant colorectal cancer cell lines. FEBS Lett 3:415–420
Erten C, Karaca B, Kucukzeybek Y, Gorumlu G, Cengiz E, Gul MK, Atmaca H, Uzunoglu S, Karabulut B, Sanli UA, Uslu R (2009) Regulation of growth factors in hormone- and drug-resistant prostate cancer cells by synergistic combination of docetaxel and octreotide. BJU Int 1:107–114
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Risbridger GP, Davis ID, Birrell SN, Tilley WD (2010) Breast and prostate cancer: more similar than different. Nat Rev Cancer 3:205–212
Watt HL, Kumar U (2006) Colocalization of somatostatin receptors and epidermal growth factor receptors in breast cancer cells. Cancer Cell Int 6:5
Reed JC (2006) Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol 7:388–398
Zhu S, Oremo JA, Li S, Zhen M, Tang Y, Du Y (2014) Synergistic antitumor activities of docetaxel and octreotide associated with apoptotic-upregulation in castration-resistant prostate cancer. PLoS One 3:e91817
Lo Nigro C, Maffi M, Fischel JL, Formento P, Milano G, Merlano M (2008) The combination of docetaxel and the somatostatin analogue lanreotide on androgen-independent docetaxel-resistant prostate cancer: experimental data. BJU Int 5:622–627
Eliopoulos AG, Kerr DJ, Herrod J (1995) The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and bcl-2. Oncogene 11:1217–1228
Daveraux QL, Takahashi R, Saivesen QS (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300–303
Roy N, Deveraux QL, Takahashi R (1997) The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J 16:6914–6925
Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G (1999) Mitochondrial membrane permeabilization during the apoptotic process. Ann NY Acad Sci 887:18–30
Berchem GJ, Bosseler M, Mine N, Avalosse B (1999) Nanomolar range docetaxel treatment sensitizes MCF-7 cells to chemotherapy induced apoptosis, induces G2 M arrest and phosphorylates bcl-2. Anticancer Res 1:535–540
Theodoropoulou M, Stalla GK (2013) Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol 3:228–252
Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed J (1998) IAP Family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58:5315–5320
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed J (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 17:2215–2223
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaca, B., Degirmenci, M., Ozveren, A. et al. Docetaxel in combination with octreotide shows synergistic apoptotic effect by increasing SSTR2 and SSTR5 expression levels in prostate and breast cancer cell lines. Cancer Chemother Pharmacol 75, 1273–1280 (2015). https://doi.org/10.1007/s00280-015-2756-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2756-1