Abstract
Purpose
Phosphatidylinositol-3-kinase I (PI3K) inhibition sensitizes a wide range of cancer cell lines to platinum/taxane-based chemotherapy. This phase I study combines buparlisib, a pan-class 1A PI3K inhibitor, with two schedules of carboplatin and paclitaxel for patients with advanced solid tumors (ClinicalTrials.gov, NCT01297452).
Methods
There were two regimens: Group 1 received carboplatin AUC 5 and paclitaxel 175 mg/m2, on day 1 of a 21-day cycle with pegfilgrastim support; Group 2 received carboplatin AUC 5 (day 1) and paclitaxel 80 mg/m2 (days 1, 8, and 15) on a 28-day cycle without growth factor support. In both groups, three dose levels of buparlisib were explored: 50, 80, and 100 mg/day. Primary endpoint was to identify recommended phase II doses of buparlisib in both groups.
Results
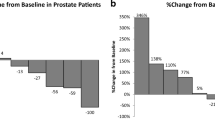
Thirty subjects enrolled, 16 in Group 1 and 14 in Group 2. The DLTs were elevated alkaline phosphatase (n = 1) and uncomplicated neutropenia (n = 2). The median numbers of cycles were 5 (Group 1) and 6 (Group 2). The MTDs for buparlisib were 100 mg/day in Group 1 and 80 mg/day in Group 2. Among 25 patients with measurable disease, the confirmed objective response rate was 20 % (one complete response, four partial responses). Among three patients with known loss of PTEN expression, all derived clinical benefit from treatment.
Conclusion
The addition of buparlisib to carboplatin + paclitaxel was well tolerated, and preliminary activity was notable against tumors with loss of PTEN expression.

Similar content being viewed by others
References
Courtney KD, Corcoran RB, Engelman JA (2010) The PI3K pathway as drug target in human cancer. J Clin Oncol 28:1075–1083
Janku F, Wheler JJ, Naing A, Falchook GS, Hong DS, Stepanek VM, Fu S, Piha-Paul SA, Lee JJ, Luthra R, Tsimberidou AM, Kurzrock R (2013) PIK3CA mutation H1047R is associated with response to PI3K/Akt/MTOR signaling pathway inhibitors in early phase clinical trials. Cancer Res 73:276–284
Ihle NT, Williams R, Chow S, Chew W, Beggrenm MI, Paine-Murrieta G, Minion DJ (2004) Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther 3:763–772
Brognard J, Clark AS, Ni Y, Dennis PA (2001) Akt/Protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61:3986–3997
Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB (2002) Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res 62:1087–1092
Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann SM, Frisch C, Dorsch M, Chene P, Shoemaker K, De Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson C, Schlegal R, Hoffman F, Garcia-Echeverria C, Sellers WR, Voliva CF (2012) Identification and characterization of NVP-BKM120, and orally available pan class I PI3-Kinase inhibitor. Mol Cancer Ther 11:317–328
Bendell JC, Rodon J, Burris HA, De Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, Goldbrunner M, Baselga J (2012) Phase I, dose-escalation study of BKM120, and oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 30:282–290
Rodon J, Brana I, Siu LI, de Jonge MJ, Homji N, Mills D, Di Tomaso E, Sarr C, Trandafir L, Massacesi C, Eskens F, Bendell JC (2014) Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs [Epub ahead of print]
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613
Spitzer RL, Kroenke K, Williams JB, Lowe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166:1092–1097
Vakiani E, Janakiraman M, Shen R, Sinha R, Zeng Z, Shia J, Cercek A, Kemeny N, D’Angelica M, Viale A, Heguy A, Paty P, Chan TA, Saltz LB, Weiser M, Solit DB (2012) Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol 30:2956–2962
Janakiraman M, Vakiani E, Zeng Z, Pratilas CA, Taylor BS, Chitale D, Halilovic E, Wilson M, Huberman K, Ricarte-Filho JC, Persaud Y, Levine DA, Fagin JA, Jhanwar SC, Mariadason JM, Lash A, Ladanyi M, Saltz LB, Heguy A, Paty SB, Solit DB (2010) Genomic and biologic characterization of exon 4 KRAS mutations in human cancer. Cancer Res 70:5901–5911
Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman-Davis N, Hollywood E, Shia J, Schwartz J, Chandrawansa K, Dontabhaktuni A, Youssoufian H, Solit DB, Saltz LB (2010) Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol 28:4240–4246
Sakr RA, Barbashina V, Morrogh M, Chandarlapaty S, Andrade VP, Arroyo CD, Olvera N, King TA (2010) Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol 18:371–374
Rustin G, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, Eisenhauer E, Sagae S, Greven K, Vergote I, Cervantes A, Vermorken J (2004) Re: new guidelines to evaluate response to treatment in solid tumors (ovarian cancer). J Natl Cancer Inst 96:487–488
Andre F, Campone M, O’Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay S, Soria J-C, Naughton M, Hurvitz SA (2010) Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol 28:5110–5115
Campone M, Levy V, Bourbouloux E, Riguad DB, Bootle D, Dutreix C, Zoellner U, Shand N, Calvo F, Raymond E (2009) Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumors. Br J Cancer 100:315–321
Kollmannsberger C, Hirte H, Siu LL, Mazurka J, Chi K, Elit L, Walsh W, Sederias J, Doyle A, Eisenhauer EA, Oza AM (2012) Temsirolimus in combination with carboplatin and paclitaxel in patients with advanced solid tumors: a NCIC-CTG, phase 1, open-label dose-escalation study (IND 179). Ann Oncol 23:238–244
Moulder S, Gladish G, Ensor J, Gonzalez-Angulo AM, Cristofanillo M, Murray JM, Booser D, Giordano SH, Brewster A, Moore J, Rivera E, Hortobagyi GN, Tran HT (2012) A phase 1 study of weekly everolimus (RAD001) in combination with docetaxel in patients with metastatic breast cancer. Cancer 118:2378–2384
Ramalingam SS, Harvey D, Saba N, Owonikoko TK, Kauh J, Shin DM, Sun S-Y, Strychor S, Tighiouart M, Egorin M, Fu H, Khuri FR (2010) Phase I and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel in recurrent/refractory nonsmall cell lung cancer. Cancer 116:3903–3909
Fury MG, Sherman E, Ho A, Katabi N, Sima C, Kelly KW, Nwankwo O, Haque S, Pfister DG (2012) A phase I study of temsirolimus + carboplatin + paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC). Cancer Chemother Pharmacol 70:121–128
Fury MG, Sherman EJ, Ho AL, Xiao H, Tsai F, Nwankwo OG, Sima CS, Heguy A, Katabi N, Haque S, Pfister D (2013) A phase I study of everolimus + docetaxel + cisplatin as induction chemotherapy for patients with locally and/or regionally advanced head and neck cancer. Cancer 119:1823–1831
Rodon J, Juric D, Gonzalez-Angulo A-M, Bendell J, Berlin J, Bootle D, Gravelin K, Huang A, Derti A, Lehar J, Wuerthner J, Boehm M, van Allen E, Wagle N, Garraway LA, Yelensky R, Stephens PJ, Miller VA, Schlegel R, Quadt C, Baselga J (2013) Towards defining the genetic framework for clinical response to treatment with BYL719, a PI3K alpha specific inhibitor. Cancer Res 73 (8 Suppl. 1): Abstract LB-65
Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, Kauffman A, Guthy D, Erdmann D, De Pover A, Furet P, Gao H, Ferretti S, Wang Y, Trappe J, Brachmann SM, Maira SM, Wilson C, Boehm M, Garcia-Echeverria C, Chene P, Wiesmann M, Cozens R, Lehar J, Schlegel R, Caravatti G, Hoffman F, Sellers WR (2014) Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of patient stratification for clinical trials. Mol Cancer Ther 13:1117–1129
Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, Bull R, Do S, Dotson J, Dudley D, Edgar KA, Friedman LS, Goldsmith R, Heald RA, Kolesnikov A, Lee L, Lewis C, Nannini M, Nonomiya J, Pang J, Price S, Prior WW, Salphati L, Sideris S, Wallin JJ, Wang L, Wei B, Sampath D, Olivero AG (2013) Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1.4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor efficacy. J Med Chem 56:4597–4610
Wee S, Wiederschain D, Maira S-M, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao Y-M, Lengauer C (2008) PTEN deficient tumors depend of PIK3CB. Proc Natl Acad Sci USA 105:13057–13062
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism, and tumorigenesis. Nature 454:776–779
Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V (2013) Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341:395–399
Acknowledgments
They also thank Saiprasad Boddu, of Sai Life Sciences, for pharmacokinetic analyses. ClinicalTrials.gov identifier: NCT01297452. The study site received funding from Novartis Pharmaceuticals.
Conflict of interest
M.F., A.H., R.C., M.L, and M.V. have served on advisory boards and/or consulted for Novartis. No potential conflict of interest was disclosed by the other authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinicaltrials.gov ID NCT01297452.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hyman, D.M., Snyder, A.E., Carvajal, R.D. et al. Parallel phase Ib studies of two schedules of buparlisib (BKM120) plus carboplatin and paclitaxel (q21 days or q28 days) for patients with advanced solid tumors. Cancer Chemother Pharmacol 75, 747–755 (2015). https://doi.org/10.1007/s00280-015-2693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2693-z