Abstract
Purpose
Early administration of both epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) monotherapy and cytotoxic chemotherapy is crucial for non-small cell lung cancer (NSCLC) patients harboring EGFR mutations. We investigated the effect of first-line administration of these therapies on subsequent therapy in NSCLC patients.
Methods
This study enrolled 63 consecutive patients with advanced EGFR-mutant NSCLC and good performance status (PS) and who underwent first-line EGFR-TKI therapy or standard cytotoxic chemotherapy and then had progressive disease, from 2007 to 2011. The ability of each patient to receive the other therapy after first-line treatment failure was assessed.
Results
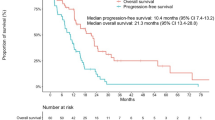
In the first-line setting, 23 and 40 patients received EGFR-TKI therapy and cytotoxic chemotherapy, respectively. At relapse, the EGFR-TKI therapy group showed more frequent PS deterioration (p = 0.042) and greater likelihood of symptomatic central nervous system (CNS) relapse (p = 0.093) compared with the cytotoxic chemotherapy group. Nine (39 %) of 23 patients initially receiving EGFR-TKI therapy could not receive standard cytotoxic therapy after progression mainly due to symptomatic CNS relapse. Only one (3 %) of 40 initially treated with cytotoxic chemotherapy failed to receive subsequent EGFR-TKI therapy (p < 0.001). Multivariate analysis revealed a correlation between the first-line therapy and the failure to switch to the other therapy after disease progression (OR 48.605, p = 0.005).
Conclusion
In this study, patients who could not receive both EGFR-TKI therapy and cytotoxic chemotherapy in the early-line setting were included more in the first-line EGFR-TKI group, suggesting a potential risk associated with missing the timing of administration of subsequent therapy. Further investigation is warranted to detect their pretreatment clinical or molecular characteristics for development of a new treatment strategy specific for such subpopulation.

Similar content being viewed by others
References
Hotta K, Matsuo K, Ueoka H et al (2004) Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 22:3852–3859
Hotta K, Matsuo K et al (2007) Long-standing debate on cisplatin- versus carboplatin-based chemotherapy in the treatment of advanced non-small cell lung cancer. J Thorac Oncol 2:96
Hotta K, Fujiwara Y, Matsuo K et al (2007) Recent improvement in the survival of patients with advanced nonsmall cell lung cancer enrolled in phase III trials of first-line, systemic chemotherapy. Cancer 109:939–948
Gridelli C et al (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 91:66–72
Gridelli C, Perrone F, Gallo C et al (2003) Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 95:362–372
Kudoh S, Takeda K, Nakagawa K et al (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663
Mok TS, Wu Y-L, Thongprasert S et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Oizumi S, Kobayashi K, Inoue A et al (2012) Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 trial. Oncologist 17:863–870
Azzoli CG, Temin S, Aliff T et al (2011) 2011 focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 29:3825–3831
Yoshioka H, Hotta K, Kiura K et al (2010) A phase II trial of erlotinib monotherapy in pretreated patients with advanced non-small cell lung cancer who do not possess active EGFR mutations: Okayama Lung Cancer Study Group trial 0705. J Thorac Oncol 5:99–104
Umemura S, Tsubouchi K, Yoshioka H et al (2012) Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer 77:134–139
Satouchi M, Ichinose Y, Ohe Y et al (2012) Final analysis of overall survival in the IPASS, an international multicenter phase III study on gefitinib and carboplatin/paclitaxel for treatment-naive NSCLC patients. Jpn J Lung Cancer 52:153–160 (in Japanese)
Fukuoka M, Wu YL, Thongprasert S et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29:2866–2874
Inoue A, Kobayashi K, Maemondo M et al (2013) Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin–paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 24:54–59
Han JY, Park K, Kim SW et al (2012) First-SIGNAL: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30:1124–1128
Mitsudomi T, Morita S, Yatabe Y et al (2012) Updated overall survival results of WJTOG 3405, a randomized phase trial comparing gefitinib with cisplatin plus docetaxel as the fine treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor(EGFR). J Clin Oncol 30(suppl). Abstr 7521
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomized, phase 3 study. Lancet Oncol 12:735–742
Addeo R, Zappavigna S, Luce A et al (2013) Chemotherapy in the management of brain metastases: the emerging role of fotemustine for patients with melanoma and NSCLC. Expert Opin Drug Saf 12:729–740
Postmus PE, Smit EF (1999) Chemotherapy for brain metastases of lung cancer: a review. Ann Oncol 19:753–759
Ceresoli GL, Cappuzzo F, Gregorc V et al (2004) Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 15:1042–1047
Hotta K, Kiura K, Ueoka H et al (2004) Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer 46:255–261
Jackman DM, Holmes AJ, Lindeman N et al (2006) Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 24:4517–4520
Baselga J, Rischin D, Ranson M et al (2002) Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol 20:4292–4302
Fukuhara T, Saijo Y, Sakakibara T et al (2008) Successful treatment of carcinomatous meningitis with gefitinib in a patient with lung adenocarcinoma harboring a mutated EGF receptor gene. Tohoku J Exp Med 214:359–363
Acknowledgments
The interpretation and reporting of these data are the sole responsibility of the authors. YK and KH had full access to all of the data in the study, were responsible for the integrity of the data and accuracy of the data analysis, and contributed to the study design, data collection, analyses, and manuscript writing. EI, NN, and TK contributed to the data collection and manuscript writing. All other co-authors contributed to the manuscript writing.
Conflict of interest
K.H. has received honoraria from Pfizer, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, and Sanofi-Aventis. N.T. has received honoraria from Chugai Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, and Sanofi-Aventis. T.K. has received honoraria from Pfizer, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Kyowa Hakko Kirin, and AstraZeneca. N.N. has received honoraria from Pfizer, Daiichi-Sankyo Pharmaceutical, Kyowa Hakko Kirin, AstraZeneca, Dainippon Sumitomo Pharmaceutical, Sanofi-Aventis, Eli Lilly Japan, Taiho Pharmaceutical, and Chugai Pharmaceutical. M.T. has received honoraria from Pfizer, Eli Lilly Japan, Taiho Pharmaceutical, and Chugai Pharmaceutical. K.K. has received honoraria from Eli Lilly Japan, Nihon Kayaku, AstraZeneca, Daiichi-Sankyo Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, and Sanofi-Aventis. All other authors declared no conflicts of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kato, Y., Hotta, K., Takigawa, N. et al. Factor associated with failure to administer subsequent treatment after progression in the first-line chemotherapy in EGFR-mutant non-small cell lung cancer: Okayama Lung Cancer Study Group experience. Cancer Chemother Pharmacol 73, 943–950 (2014). https://doi.org/10.1007/s00280-014-2425-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2425-9