Abstract
Purpose
Erlotinib, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy) quinazolin-4-amine is approved for the treatment for non-small cell lung cancer and pancreatic cancer. Because erlotinib is metabolized predominately by CYP3A4, co-administration of compounds that increase CYP3A4 activity may alter the efficacy and safety of erlotinib therapy. Two phase I studies were conducted in healthy male subjects to evaluate the effect of pre- or co-administered rifampicin, a CYP3A4 inducer, on the pharmacokinetics of erlotinib.
Methods
Study 1 included Groups A (erlotinib 150 mg days 1 and 15, rifampicin 600 mg days 8–14) and B (erlotinib 150 mg days 1 and 15) in a parallel group study design. Study 2 subjects received erlotinib 150 mg day 1, erlotinib 450 mg day 15, and rifampicin 600 mg days 8–18. The primary endpoint in each study was the ratio of exposure (AUC0–∞ and C max) between days 1 and 15. Urinary cortisol metabolic induction ratios were determined in Study 1 for Group A subjects only.
Results
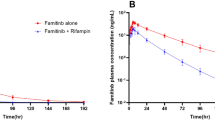
In Study 1, the geometric mean ratios of AUC0–∞ and C max were 33 and 71 %, respectively, and the mean cortisol metabolic index increased from 7.4 to 27.0, suggesting cytochrome P450 (CYP) enzyme induction. In Study 2, the geometric mean ratios for AUC0–∞ and C max were 19 and 34 % (when dose adjusted from 450 to 150 mg erlotinib), respectively, a greater relative decrease than observed in Study 1.
Conclusions
Erlotinib exposure (AUC0–∞ and C max) was reduced after pre- or concomitant dosing with rifampicin. Doses of ≥450 mg erlotinib may be necessary to compensate for concomitant medications with strong CYP3A4 enzyme induction effect.


Similar content being viewed by others
References
Shepherd FA, Rodrigues PJ, Ciuleanu T et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353(2):123–132
Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966
Cappuzzo F, Ciuleanu T, Stelmakh L et al (2010) Erlotinib as maintenance treatment in advanced non-small cell lung cancer: a multicentre, randomised, placebo controlled phase 3 study. Lancet Oncol 11(6):521–529
Tarceva® Full Prescribing Information, Astellas Pharma US, Inc., and Genentech, Inc, May 2013
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
National Comprehensive Cancer Network (2013) NCCN Clinical practice Guidelines in Oncology; Non-Small-Cell Lung Cancer Version 2.2013. http://www.nccn.com
Hamilton M, Wolf JL, Rusk J et al (2006) Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 12(7 Pt 1):2166–2171
Rakhit A, Pantze MP, Fettner S, Jones HM, Charoin JE, Reik M et al (2008) The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer based simulation (SimCYP™) predicts in vivo metabolic inhibition. Eur J Clin Pharmacol 64:31–41
Calvert H, Twelves C, Ranson M, Anthoney A, Plummer R, Fettner S et al. (2005) The effect of erlotinib (Tarceva™) on CYP3A4 activity, as quantified by the erythromycin breath test and oral midazolam kinetics in cancer patients—preliminary results. J Clin Oncol ASCO Ann Mtg Proceed 23(16s, Part I of II): 3076
Ohnhaus E, Breckenridge AM, Park BK (1989) Urinary excretion of 6β-hydroxycortisol and the time course measurement of enzyme induction in man. Eur J Clin Pharmacol 36:39–46
Ling J, Johnson KA, Miao Z, Rakhit A, Pantze MP, Hamilton M et al (2006) Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos 34(3):420–426
Tanaka C, Yin OQ, Smith T et al (2011) Effects of rifampin and ketoconazole on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol 51(1):75–83
Yeo KP, Lowe SL, Lim MT et al (2005) Pharmacokinetics of ruboxistaurin are significantly altered by rifampicin-mediated CYP3A4 induction. Br J Clin Pharmacol 61(2):200–210
Saima S, Furuie K, Yoshimoto H et al (2002) The effects of rifampicin on the pharmacokinetics and pharmacodynamics of orally administered nilvadipine to healthy subjects. Br J Clin Pharmacol 53(2):203–206
Israili ZH, Rogers CM, El-Attar H (1987) Pharmacokinetics of antituberculosis drugs in patients. J Clin Pharmacol 27:78–83
Correia MA (2003) Hepatic cytochrome P450 degradation: mechanistic diversity of the cellular sanitation brigade. Drug Metab Rev 35(2 & 3):107–143
Reitman ML, Chu X, Cai X et al (2011) Rifampin’s acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther 89:234–242
O’Reilly RA (1975) Interaction of chronic daily warfarin therapy and rifampicin. Ann Intern Med 83:506–508
Lam JL, Shugarts SB, Okochi H, Benet LZ (2006) Elucidating the effect of final-day dosing of rifampin in induction studies on hepatic drug disposition and metabolism. J Pharmacol Exp Ther 319(2):864–870
Kirby BJ, Collier AC, Kharasch ED et al (2012) Complex drug interactions of the HIV protease inhibitors 3: effect of simultaneous or staggered dosing of digoxin and ritonavir, nelfinavir, rifampin, or bupropion. Drug Metab Dispos 40:610–616
Guidance for industry: drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations Draft Guidance February 2012. Accessed 9 Apr 2013 http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
Elmeliegy MA, Carcaboso AM, Tagen M et al (2011) Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res 17(1):89–99
Li Y, Xu J, Lai G et al (2012) Metabolic switching of BILR 355 in the presence of ritonavir. Drug Metab Dispos 40(6):1130–1137
Shi Z, Peng XX, Kim IW et al (2007) Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res 67(22):11012–11020
Li J, Cusatis G, Brahmer J (2007) Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther 6(3):432–438
Hidalgo M, Siu LL, Nemunaitis J et al (2001) Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19(13):3267–3279
Prados MD, Lamborn KR, Chang S et al (2006) Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol 8(1):67–78
Abbas R, Fettner S, Riek M, Bulsara S, Pantze M, Hamilton M, Frohna P, Rakhit A (2003) A drug-drug interaction study to evaluate the effect of rifampicin on the pharmacokinetics of the EGF receptor tyrosine kinase inhibitor, erlotinib, in healthy subjects. Proc Am Soc Clin Oncol 22:137 (abstr 548)
Acknowledgments
Funding for the clinical studies described was provided by F-Hoffman La Roche (Study 1) and OSI Pharmaceuticals Inc (Study 2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamilton, M., Wolf, J.L., Drolet, D.W. et al. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol 73, 613–621 (2014). https://doi.org/10.1007/s00280-014-2390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2390-3