Abstract
Purpose
Use of the fourth-generation oral fluoropyrimidine S-1 together with gemcitabine has shown striking anticancer effects. In this single-arm phase I trial of preoperative combination therapy using gemcitabine and S-1 concurrently with radiotherapy, we verified the safety and feasibility and determined the maximum-tolerated dose of each drug in patients with resectable pancreatic cancer.
Methods
A standard 3+3 dose escalation scheme was used. Patients with cytologically or histologically proven resectable pancreatic ductal adenocarcinoma were administered 30-min intravenous gemcitabine infusions on days 1, 8, 22, and 29 and S-1 orally on days 1–5, 8–12, 22–26, and 29–33. A total radiation dose of 50.4 Gy (1.8 Gy/day, 5 times per week, 28 fractions) was concurrently delivered. Surgical exploration was scheduled 4–7 weeks after the final radiation fraction.
Results
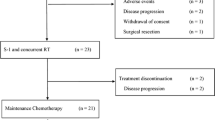
Twenty-one patients were enrolled. No treatment-related deaths occurred during this study. Recommended doses were determined to be 80 mg/m2 of S-1 daily and 1,000 mg/m2 of gemcitabine. CA19-9 was reduced to <50 % of baseline values in 12 (75 %) of 16 measurable patients. Nineteen of 21 enrolled patients successfully underwent surgical resection.
Conclusions
Preoperative chemoradiotherapy consisting of gemcitabine and S-1 concurrent with full-dose radiation is feasible and well tolerated.

Similar content being viewed by others
Abbreviations
- PDAC:
-
Pancreatic ductal adenocarcinoma
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- MTD:
-
Maximum-tolerated dose
- RD:
-
Recommended dose
- DLT:
-
Dose-limiting toxicity
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62(1):10–29. doi:10.3322/caac.20138
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350(12):1200–1210. doi:10.1056/NEJMoa032295
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297(3):267–277. doi:10.1001/jama.297.3.267
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Olah A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Buchler MW (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304(10):1073–1081. doi:10.1001/jama.2010.1275
Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M, Shimada M, Kanemitsu K (2009) A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer 101(6):908–915. doi:10.1038/sj.bjc.6605256
Eguchi H, Nagano H, Tanemura M, Takeda Y, Marubashi S, Kobayashi S, Kawamoto K, Wada H, Hama N, Akita H, Mori M, Doki Y (2013) Preoperative chemoradiotherapy, surgery and adjuvant therapy for resectable pancreatic cancer. Hepato-gastroenterology 60(126). doi:10.5754/hge12974
Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H, Tomita Y, Nishiyama K (2009) Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg 250(1):88–95. doi:10.1097/SLA.0b013e3181ad65cc
Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA (2008) Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 26(21):3496–3502. doi:10.1200/jco.2007.15.8634
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820. doi:10.1056/NEJMoa072252
Nakai Y, Isayama H, Sasaki T, Sasahira N, Ito Y, Kogure H, Togawa O, Matsubara S, Arizumi T, Yagioka H, Yashima Y, Kawakubo K, Mizuno S, Yamamoto K, Hirano K, Tsujino T, Ijichi H, Tateishi K, Toda N, Tada M, Omata M, Koike K (2010) Impact of S-1 on the survival of patients with advanced pancreatic cancer. Pancreas 39(7):989–993. doi:10.1097/MPA.0b013e3181d91936
Schultheis B, Strumberg D, Bergmann L, Graeven U, Hanauske AR, Lipp R, Schuette J, Saito K, Scigalla P, Scheulen ME (2012) Results of a phase II trial of S-1 as first-line treatment of metastatic pancreatic cancer (CESAR-study group). Invest New Drugs 30(3):1184–1192. doi:10.1007/s10637-011-9665-x
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, Shimamura T, Sho M, Kitano M, Cheng AL, Mizumoto K, Chen JS, Furuse J, Funakoshi A, Hatori T, Yamaguchi T, Egawa S, Sato A, Ohashi Y, Okusaka T, Tanaka M (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31(13):1640–1648. doi:10.1200/jco.2012.43.3680
Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, Kogure H, Matsubara S, Ito Y, Togawa O, Arizumi T, Hirano K, Tada M, Omata M, Koike K (2012) A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 106(12):1934–1939. doi:10.1038/bjc.2012.183
Ozaka M, Matsumura Y, Ishii H, Omuro Y, Itoi T, Mouri H, Hanada K, Kimura Y, Maetani I, Okabe Y, Tani M, Ikeda T, Hijioka S, Watanabe R, Ohoka S, Hirose Y, Suyama M, Egawa N, Sofuni A, Ikari T, Nakajima T (2012) Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol 69(5):1197–1204. doi:10.1007/s00280-012-1822-1
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Buchler MW (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142(5):761–768. doi:10.1016/j.surg.2007.05.005
Hoffman JP, Lipsitz S, Pisansky T, Weese JL, Solin L, Benson AB 3rd (1998) Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol 16(1):317–323
Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Kamata M, Ohe C, Sakaida N, Uemura Y, Kitade H, Tanigawa N, Inoue K, Matsui Y, Kwon AH (2012) Neo-adjuvant chemoradiation therapy using S-1 followed by surgical resection in patients with pancreatic cancer. J Gastrointest Surg 16(4):784–792. doi:10.1007/s11605-011-1795-0
Takahashi H, Ohigashi H, Ishikawa O, Gotoh K, Yamada T, Nagata S, Tomita Y, Eguchi H, Doki Y, Yano M (2012) Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine-based chemoradiation therapy for resectable pancreatic cancer. Ann Surg 255(1):95–102. doi:10.1097/SLA.0b013e31823d813c
Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ, Ames FC (1992) Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 127(11):1335–1339
Chatterjee D, Katz MH, Rashid A, Varadhachary GR, Wolff RA, Wang H, Lee JE, Pisters PW, Vauthey JN, Crane C, Gomez HF, Abbruzzese JL, Fleming JB (2012) Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 118(12):3182–3190. doi:10.1002/cncr.26651
White RR, Xie HB, Gottfried MR, Czito BG, Hurwitz HI, Morse MA, Blobe GC, Paulson EK, Baillie J, Branch MS, Jowell PS, Clary BM, Pappas TN, Tyler DS (2005) Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol 12(3):214–221. doi:10.1245/aso.2005.03.105
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825. doi:10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. doi:10.1056/NEJMoa1304369
Matsumura Y, Suyama M, Sai J, Kubokawa Y, Kamiya T, Watanabe S (2010) A phase 1 study of gemcitabine and S-1 concurrent radiotherapy in patients with locally advanced pancreatic cancer. Suizo 25(2):109–116
Sho M, Akahori T, Tanaka T, Kinoshita S, Tamamoto T, Nomi T, Yamato I, Hokuto D, Yasuda S, Kawaguchi C, Nishiofuku H, Marugami N, Enomonoto Y, Kasai T, Hasegawa M, Kichikawa K, Nakajima Y (2013) Pathological and clinical impact of neoadjuvant chemoradiotherapy using full-dose gemcitabine and concurrent radiation for resectable pancreatic cancer. J Hepatobiliary Pancreat Sci 20(2):197–205. doi:10.1007/s00534-012-0532-8
Desai SP, Ben-Josef E, Normolle DP, Francis IR, Greenson JK, Simeone DM, Chang AE, Colletti LM, Lawrence TS, Zalupski MM (2007) Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol 25(29):4587–4592. doi:10.1200/jco.2007.12.0592
Harada K, Kawaguchi S, Supriatno, Onoue T, Yoshida H, Sato M (2004) Combined effects of the oral fluoropyrimidine anticancer agent, S-1 and radiation on human oral cancer cells. Oral Oncol 40(7):713–719. doi:10.1016/j.oraloncology.2004.01.013
Conflict of interest
None.
Funding
This study was supported in part by Japanese Foundation for Multidisciplinary Treatment of Cancer.
Author information
Authors and Affiliations
Corresponding author
Additional information
UMIN Clinical Trials Registry Number: 000002649.
Rights and permissions
About this article
Cite this article
Eguchi, H., Nagano, H., Kobayashi, S. et al. A phase I trial of combination therapy using gemcitabine and S-1 concurrent with full-dose radiation for resectable pancreatic cancer. Cancer Chemother Pharmacol 73, 309–315 (2014). https://doi.org/10.1007/s00280-013-2357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2357-9