Abstract
Purpose
A phase 1 study evaluated the QTc prolongation potential of siltuximab, a chimeric, anti-interleukin-6 mAb, in patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), or low-volume MM.
Methods
Patients with baseline QTcF and QTcB ≤ 500 ms, QRS < 100 ms, PR < 200 ms and no significant cardiac disease received siltuximab 15 mg/kg q3w, the highest dosage used in clinical studies, for 4 cycles. Twelve-lead ECGs obtained at multiple time points pre- and post-infusion at cycles 1 and 4 were evaluated by central cardiology laboratory. No effect on QTc interval was concluded if the upper limit of least square (LS) mean 90 % CI for QTc change from baseline at each time point was <20 ms.
Results
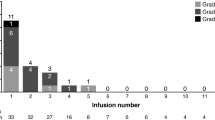
An effect on QTc prolongation was ruled out, as the upper bound of 90 % CI was <10 ms at each time point in 27 evaluable patients (13 MGUS, 13 SMM, 1 low-volume MM) with no differences between disease types. Maximum mean QTc increase from baseline occurred 3 h after cycle 1 infusion (QTcF = 3.2 [LS mean 90 % CI −0.01, 6.45] ms; QTcB = 2.7 [−0.69, 6.14] ms). At all other time points, mean QTcF and QTcB increase from baseline was ≤1.5 ms and upper bound 90 % CI was ≤5.1 ms. Twenty patients had mostly low-grade AEs, including nausea, fatigue (20 % each); thrombocytopenia, headache (each 13 %); dyspnea, leukopenia, neutropenia, paresthesia, abnormal hepatic function, URTI (each 10 %). Three MGUS patients achieved 50 % M-protein reduction. There was no association between siltuximab pharmacokinetics and QTc interval.
Conclusions
Siltuximab did not affect the QTc interval. Overall safety was similar to other single-agent siltuximab studies.


Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV (2009) Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 113(22):5412–5417. doi:10.1182/blood-2008-12-194241
Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF, Jelinek DF, Fonseca R, Melton LJ 3rd, Rajkumar SV (2007) Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 356(25):2582–2590. doi:10.1056/NEJMoa070389
Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kroger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M (2010) Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24(6):1121–1127. doi:10.1038/leu.2010.60
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23(1):3–9. doi:10.1038/leu.2008.291
Mateos MV, Lopez-Corralo L, Hernadez M, Giraldo P, De La Rubia J, de Arriba F, Rosinol L, Lahuerta JJ, Palomera L, Bargary J, Oriol A, Prosper F, Lopez J, Olavarria E, Martino ML, Teruel A, Hernandex JM (2011) Smoldering multiple myeloma (SMM) at high-risk of progression to symptomatic disease: a phase III, randomized, multicenter trial based on lenalidomide–dexamethasone (Len–Dex) as induction therapy followed by maintenance therapy with len alone vs no treatment. Blood 118 (ASH Annual meeting abstracts): abstract 991
Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, Geyer SM, Campbell ME, Kyle RA, Rajkumar SV, Greipp PR, Kline MP, Xiong Y, Moon-Tasson LL, Donovan KA (2009) Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc 84(2):114–122. doi:10.4065/84.2.114
Hilbert DM, Kopf M, Mock BA, Kohler G, Rudikoff S (1995) Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med 182(1):243–248
Seideman J, Peritt D (2002) A novel monoclonal antibody screening method using the Luminex-100 microsphere system. J Immunol Methods 267(2):165–171
Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, Borghaei H, Jagannath S, Sokol L, Usmani SZ, van de Velde H, Qin X, Puchalski TA, Hall B, Reddy M, Qi M, van Rhee F (2013) A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res 19(13):3659–3670. doi:10.1158/1078-0432.CCR-12-3349
van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, Sokol L, Crawford J, Cornfeld M, Qi M, Qin X, Herring J, Casper C, Kurzrock R (2010) Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol 28(23):3701–3708. doi:10.1200/JCO.2009.27.2377
Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B (2010) A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer 103(8):1154–1162. doi:10.1038/sj.bjc.6605872
Angevin E, Elez E, Cohen SJ, van laethem J-L, Ottensmeier C, Joly F, Ray-Coquard I, Lopez-Martin JA, Dirix L, Machiels J-P, Clive S, Steven NM, Reddy M, Hall B, Puchalski TA, Bandekar R, Van de Velde H, Tromp BJ, Vermeulen J, Kurzrock R (2012) Phase I/II, two-part, open-label, multiple-dose, dose-escalation study of siltuximab in patients with solid tumors. J Clin Oncol 30(15 Suppl):2583
Grange S, Schmitt C, Gerogy A, Ludger B, Kuhn B, Zhang X (2009) A clinical study to assess the effect of tocilizumab at a therapeutic dose and a supra-therapeutic dose of tocilizumab on QT/QTc interval after a single dose in healthy subjects. Arthritis Rheum 60(Suppl 10):1614. doi:10.1002/art.26688
Greipp PR (2000) Smoldering, asymptomatic stage 1, and indolent myeloma. Curr Treat Options Oncol 1(2):119–126
Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM (2010) Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res 16(5):1652–1661. doi:10.1158/1078-0432.CCR-09-2581
Fridericia LS (1920) Die systolendauer im elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med Scand 53(1):469–486
Bazett HC (1919) An analysis of the time-relationship of electrocardiograms. Heart 70:353–370
International Conference on Harmonisation (2005) guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs; availability. Notice. Fed Regist 70(202):61134–61135
Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, Lentzsch S, Frank RC, Zweegman S, Wijermans PW, Orlowski RZ, Kranenburg B, Hall B, Casneuf T, Qin X, van de Velde H, Xie H, Thomas SK (2013) A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol 161(3):357–366. doi:10.1111/bjh.12266
Birmann BM, Neuhouser ML, Rosner B, Albanes D, Buring JE, Giles GG, Lan Q, Lee IM, Purdue MP, Rothman N, Severi G, Yuan JM, Anderson KC, Pollak M, Rifai N, Hartge P, Landgren O, Lessin L, Virtamo J, Wallace RB, Manson JE, Colditz GA (2012) Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood 120(25):4929–4937. doi:10.1182/blood-2012-03-417253
Acknowledgments
The authors thank Jennifer Han of Janssen Services, LLC, for providing medical writing support in developing and preparing this manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, S.K., Suvorov, A., Noens, L. et al. Evaluation of the QTc prolongation potential of a monoclonal antibody, siltuximab, in patients with monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, or low-volume multiple myeloma. Cancer Chemother Pharmacol 73, 35–42 (2014). https://doi.org/10.1007/s00280-013-2314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2314-7