Abstract
Purpose
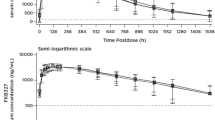
To demonstrate pharmacokinetic (PK) comparability for a single dose of 600 mg subcutaneous (SC) trastuzumab, administered via a novel single-use injection device (SID) or handheld syringe in 119 randomized healthy male subjects.
Methods
The co-primary PK endpoints area under the time–concentration curve from the start of dosing to day 22 (AUC0–21 days) and maximum observed trastuzumab serum concentration (C max) were dose-normalized and body-weight-adjusted, and compared using geometric mean ratios (GMRs). SID performance, injection site pain, adverse events, and antidrug antibodies (ADAs) were assessed.
Results
GMRs and 90 % confidence intervals (CIs) were 1.01 (0.96–1.07) for AUC0–21 days and 1.02 (0.96–1.10) for C max, which fell within the prespecified bioequivalence range (0.80–1.25). No SID quality issues or failures occurred. Adverse events were mostly mild, with no deaths, adverse event-related withdrawals, or life-threatening, cardiac, or serious events reported. The ADA rate was low, and no neutralizing antibodies were detected.
Conclusions
Trastuzumab SC via SID demonstrated comparable PK and safety to handheld syringe administration. SID performance was very satisfactory.



Similar content being viewed by others
References
Aebi S, Davidson T, Gruber G, Cardoso F, ESMO Guidelines Working Group (2011) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 22:vi12–vi24
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, ESMO Guidelines Working Group (2012) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:vii11–vii19
National Comprehensive Cancer Network (2013) NCCN clinical practice guidelines in oncology: Breast cancer V1
Herceptin: Summary of Product Characteristics (2011) available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf. Accessed 24 June 2013
Bittner B, Richter WF, Hourcade-Potelleret F, McIntyre C, Herting F, Zepeda ML, Schmidt J (2012) Development of a subcutaneous formulation for trastuzumab—nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Arzneimittelforschung 62:401–409
Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI (2006) A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release 114:230–241
Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B (2013) Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol 53:192–201
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V, Melichar B, Jackisch C (2012) Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 13:869–878
Huskisson EC (1974) Measurement of pain. Lancet 2:1127–1131
Singer AJ, Thode HC Jr (1998) Determination of the minimal clinically significant difference on a patient visual analog satisfaction scale. Acad Emerg Med 5:1007–1111
European Medicines Agency Committee for Medicinal Products for Human Use (2010) Guideline on the investigation of bioequivalence. EMA, London
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2001) Guidance for industry: statistical approaches to establishing bioequivalence. FDA, Rockville
Acknowledgments
This study was funded by F. Hoffmann-La Roche. We would like to acknowledge all investigators participating in this trial in addition to the Global Study Management team members and Luis Herráez-Baranda. We would also like to thank Marcel Both for his involvement in SID development and for providing onsite training in use of the SID. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche.
Conflict of interest
M.L. and D.H. are employees of F. Hoffmann-La Roche Ltd. R.M., C.L., and B.L.L. are employees of Genentech Inc. The remaining authors had no potential conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2013_2273_MOESM2_ESM.tif
Supplementary Fig 1 Patient disposition. AUC0–21 days, area under the time–concentration curve from start of dosing to day 22; Cday 21, concentration at day 22; Cmax, maximum serum concentration; PK, pharmacokinetics; SC, subcutaneous; SD, standard deviation; SID, single-use injection device; Tmax, time to maximum serum concentration. a Exclusion criteria met (n = 37), inclusion criteria not met (n = 22), withdrawn consent (n = 10), other (n = 1). b Ineligibility (n = 3), withdrawn consent (n = 1) (TIFF 131 kb)
280_2013_2273_MOESM3_ESM.tif
Supplementary Fig 2 Mean injection site pain assessments on 100-mm VAS. Higher scores correspond to greater pain intensity. SD, standard deviation; SID, single-use injection device; VAS, visual analog scale (TIFF 137 kb)
Rights and permissions
About this article
Cite this article
Wynne, C.J., Ellis-Pegler, R.B., Waaka, D.S. et al. Comparative pharmacokinetics of subcutaneous trastuzumab administered via handheld syringe or proprietary single-use injection device in healthy males. Cancer Chemother Pharmacol 72, 1079–1087 (2013). https://doi.org/10.1007/s00280-013-2273-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2273-z