Abstract
Purpose
Brentuximab vedotin (ADCETRIS®), an antibody–drug conjugate, comprises an anti-CD30 antibody conjugated by a protease-cleavable linker to a microtubule-disrupting agent, monomethyl auristatin E (MMAE). In vitro studies showed that MMAE does not interfere with hERG K+ channels at clinically relevant concentrations. In pivotal phase 2 clinical trials in patients with relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma, brentuximab vedotin has shown substantial efficacy and an acceptable safety profile. This phase 1 open-label study was designed to evaluate the effect of brentuximab vedotin on the duration of cardiac ventricular repolarization.
Methods
Patients with CD30-positive hematologic malignancies were treated with 1.8 mg/kg brentuximab vedotin by intravenous infusion every 3 weeks for up to 16 cycles. The primary endpoint was the change from baseline to Cycle 1 Days 2, 3, and 4 in the duration of ventricular repolarization using Fridericia’s corrected QT interval (QTcF).
Results
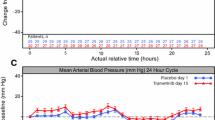
There was no clinically meaningful change from baseline in the duration of ventricular repolarization as measured by QTcF in the 46 evaluable patients out of 52 total patients treated with brentuximab vedotin. There was no evidence of treatment-emergent cardiac safety abnormalities. Brentuximab vedotin was generally well tolerated with a response rate and an adverse event profile consistent with prior studies.
Conclusion
There is no significant prolongation of the QT/QTc interval with brentuximab vedotin in patients with CD30-positive hematologic malignancies.



Similar content being viewed by others
References
Wu AM, Senter PD (2005) Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 23(9):1137–1146
Carter PJ, Senter PD (2008) Antibody-drug conjugates for cancer therapy. Cancer J 14(3):154–169
Sutherland MS, Sanderson RJ, Gordon KA et al (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem 281:10540–10547
Francisco JA, Cerveny CG, Meyer DL et al (2003) cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102:1458–1465
Food and Drug Administration, HHS (2005) International conference on harmonization; guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs; availability. Notice Fed Regist 70(202):61134–61135
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Garnett CE, Beasley N, Bhattaram VA et al (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Malik M, Camm AJ (2001) Evaluation of drug-induced QT interval prolongation: implications for drug approval and labelling. Drug Saf 24(5):323–351
Shah RR, Drug-induced QT (2010) Interval shortening: potential harbinger of proarrhythmia and regulatory perspectives. Br J Pharmacol 159(1):58–69
Pro B, Advani R, Brice P et al (2012) Brentuximab Vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma: results of a phase 2 study. J Clin Oncol 30(18):2190–2196
Younes A, Gopal AK, Smith SE et al (2012) Results of a pivotal phase II study of Brentuximab Vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30(18):2183–2189
Rothe A, Sasse S, Goergen H et al (2012) Brentuximab vedotin for relapsed or refractory CD30-positive hematologic malignancies: the GHSG experience. Blood 120(7):1470–1472
Collett D (1991) Statistical inference for binary data. In: Modelling binary data. Chapman and Hall, London, pp 23–26
Acknowledgments
Research funding for the study was provided by Seattle Genetics, Inc. and Millennium: The Takeda Oncology Company. The authors wish to acknowledge Nancy Whiting for protocol development; Elizabeth Thomas, Pamela Levine, Bess Sorensen, and Mike Thorn for statistical guidance; Dr. Tim Callahan for ECG interpretation; and Jennifer Houser for medical writing assistance, all under the sponsorship of Seattle Genetics, Inc. The authors would also like to express their gratitude to patients and their families.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, T.H., Chen, R., Advani, R. et al. Brentuximab vedotin does not cause clinically relevant QTc interval prolongation in patients with CD30-positive hematologic malignancies. Cancer Chemother Pharmacol 72, 241–249 (2013). https://doi.org/10.1007/s00280-013-2192-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2192-z