Abstract
Purpose
Paclitaxel–cisplatin (TC) combination is effective and well tolerated in patients with unresectable gastric cancer. We investigated the efficacy and safety of TC for locally advanced gastric cancers in a neoadjuvant setting.
Methods
Patients received 2–4 courses of paclitaxel (80 mg/m2) and cisplatin (25 mg/m2) on days 1, 8, and 15 in a 4-weekly schedule, followed by radical gastrectomy. Primary endpoint was the pathological response rate: percentage of tumors in which one-third or more parts were affected.
Results
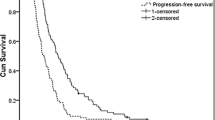
All 52 patients enrolled were eligible. Thirty-six (69.7 %) patients completed two or more courses of chemotherapy. Forty-three patients (82.7 %) underwent surgery, 33 (63.5 %) had R0 resection, and there was no treatment-related death. The pathological response was 34.6 % (95 % CI 22.0–49.1) for all registered patients; the null hypothesis of tumor response ≤10 % was rejected (p < 0.0001). The 3-year overall survival was 41.5 % (95 % CI 27.4–55.0).
Conclusions
The neoadjuvant chemotherapy with TC was safe and effective for patients with locally advanced gastric cancer, and further study is needed to confirm the effectiveness of this regimen.

Similar content being viewed by others
References
Brenner B, Shah MA, Karpeh MS, Gonen M, Brennan MF, Coit DG, Klimstra DS, Tang LH, Kelsen DP (2006) A phase II trial of neoadjuvant cisplatin–fluorouracil followed by postoperative intraperitoneal floxuridine–leucovorin in patients with locally advanced gastric cancer. Ann Oncol 17:1404–1411
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer
Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, Dieckmann C, Schoder V, Adam G (2004) Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 230:465–471
A Japanese Gastric Cancer (1998) Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1:10–24
Kobayashi M, Sakamoto J, Namikawa T, Okamoto K, Okabayashi T, Ichikawa K, Araki K (2006) Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World J Gastroenterol 12:1412–1415
Kurokawa Y, Sasako M, Ando N, Sano T, Igaki H, Iwasaki Y, Tsuburaya A, Fukuda H (2009) Validity of response criteria in neoadjuvant chemotherapy against gastric and esophageal cancer: Correlative analyses of multicenter JCOG trials. Gastrointestinal Cancers Symposium, Orland. p. Abst 11
Macdonald JS (2005) Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol 90:166–170
Nagata N, Kimura M, Hirabayashi N, Tuburaya A, Murata T, Kondo K, Fukuda Y, Kobayashi M, Miyashita Y, Nakao A, Sakamoto J (2008) Phase II study of weekly paclitaxel and cisplatin combination therapy for advanced or recurrent gastric cancer. Hepatogastroenterology 55:1846–1850
Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M (2010) Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303:1729–1737
Sakamoto J, Matsui T, Kodera Y (2009) Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer 12:69–78
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11:439–449
Tsuburaya A, Sakamoto J, Morita S, Kodera Y, Kobayashi M, Miyashita Y, Macdonald JS (2005) A randomized phase III trial of post-operative adjuvant oral fluoropyrimidine versus sequential paclitaxel/oral fluoropyrimidine; and UFT versus S1 for T3/T4 gastric carcinoma: the stomach cancer adjuvant multi-institutional trial group (Samit) trial. Jpn J Clin Oncol 35:672–675
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N (2009) Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 96:1015–1022
Yoshikawa T, Tsuburaya A, Morita S, Kodera Y, Ito S, Cho H, Miyashita Y, Sakamoto J (2010) A comparison of multimodality treatment: two or four courses of paclitaxel plus cisplatin or S-1 plus cisplatin followed by surgery for locally advanced gastric cancer, a randomized Phase II trial (COMPASS). Jpn J Clin Oncol 40:369–372
Acknowledgments
This work was supported by the non-profit organization Epidemiological & Clinical Research Information Network.
Conflict of interest
All authors declared no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuburaya, A., Nagata, N., Cho, H. et al. Phase II trial of paclitaxel and cisplatin as neoadjuvant chemotherapy for locally advanced gastric cancer. Cancer Chemother Pharmacol 71, 1309–1314 (2013). https://doi.org/10.1007/s00280-013-2130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2130-0