Abstract
Purpose
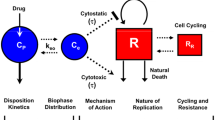
Mutations and activations of the MEK and PI3K pathways are associated with the development of many cancers. GDC-0973 and GDC-0941 are inhibitors of MEK and PI3K, respectively, currently being evaluated clinically in combination as anti-cancer treatment. The objective of these studies was to characterize the relationship between the plasma concentrations of GDC-0973 and GDC-0941 administered in combination and efficacy in A2058 melanoma xenograft.
Methods
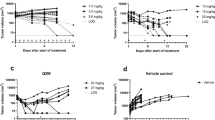
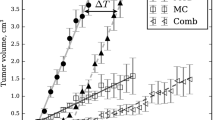
GDC-0973 and GDC-0941 were administered to A2058 tumor-bearing mice daily (QD) or every third day (Q3D) either as single agents or in combination. A semi-mechanistic population anti-cancer model was developed to simultaneously describe the tumor growth following QD/Q3D single-agent and QD combination treatments. The interaction terms ψ included in the model were used to assess whether the combination was additive. Using this model, data from the Q3D combination regimen were simulated and compared with the observed tumor volumes.
Results
The model consisting of saturable tumor growth provided the best fit of the data. The estimates for ψ were not significantly different from 1, suggesting an additive effect of GDC-0973 and GDC-0941 on tumor growth inhibition. The population rate constants associated with tumor growth inhibition for GDC-0973 and GDC-0941 were 0.00102 and 0000651 μM−1 h−1, respectively. Using the model based on single-agent and QD combination efficacy data, simulations adequately described the tumor growth from the Q3D combination regimen.
Conclusions
These findings suggest that, based on minimal data, it is possible to predict the effects of various combinations preclinically and also assess the potential clinical efficacy of combinations using human pharmacokinetic inputs.





Similar content being viewed by others
References
Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK (1998) The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA 95:14950–14955
Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K (2011) A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res 71:154–163
Bauer RJ, Guzy S (eds) (2004) Monte carlo parametric expectation maximization (MC-PEM) method for analyzing population pharmacokinetic/pharmacodynamic (PK/PD) data. Kluwer Academic Publishers, Boston
Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Benedetti Panici P, Mancuso S, Neri G, Testa JR (1995) Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer 64:280–285
Belvin M, Berry L, Chan J, Otter Dd, Friedman L, Hoeflich K, Koeppen H, Merchant M, Orr C, Rice K (2010) Intermittent dosing of the MEK inhibitor, GDC-0973, and the PI3K inhibitor, GDC-0941, results in prolonged accumulation of Bim and causes strong tumor growth inhibition in vivo. 22nd EORTC—NCI–AACR Symposium on Molecular Targets and Cancer Therapeutics Programme and Abstract Book Berlin, Germany. p 48
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Damia G, D’Incalci M (2009) Contemporary pre-clinical development of anticancer agents–what are the optimal preclinical models? Eur J Cancer 45:2768–2781
Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, Friedman LS, Belvin M (2010) Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res 70:1164–1172
Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, Friedman LS, Hayes A, Hancox TC, Kugendradas A, Lensun L, Moore P, Olivero AG, Pang J, Patel S, Pergl-Wilson GH, Raynaud FI, Robson A, Saghir N, Salphati L, Sohal S, Ultsch MH, Valenti M, Wallweber HJ, Wan NC, Wiesmann C, Workman P, Zhyvoloup A, Zvelebil MJ, Shuttleworth SJ (2008) The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin -4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 51:5522–5532
Gollob JA, Wilhelm S, Carter C, Kelley SL (2006) Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol 33:392–406
Goteti K, Garner CE, Utley L, Dai J, Ashwell S, Moustakas DT, Gonen M, Schwartz GK, Kern SE, Zabludoff S, Brassil PJ (2010) Preclinical pharmacokinetic/pharmacodynamic models to predict synergistic effects of co-administered anti-cancer agents. Cancer Chemother Pharmacol 66:245–254
Greco WR, Bravo G, Parsons JC (1995) The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385
Hoeflich KP, Merchant M, Orr C, Chan J, Den Otter D, Berry L, Kasman I, Koeppen H, Rice K, Yang NY, Engst S, Johnston S, Friedman LS, Belvin M (2012) Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res 72:210–219
Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, Januario T, Savage H, Punnoose E, Truong T, Zhou W, Berry L, Murray L, Amler L, Belvin M, Friedman LS, Lackner MR (2009) In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 15:4649–4664
Koch G, Walz A, Lahu G, Schropp J (2009) Modeling of tumor growth and anticancer effects of combination therapy. J Pharmacokinet Pharmacodyn 36:179–197
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947
Madhunapantula SV, Robertson GP (2008) Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res 68:5–8
Mager DE, Woo S, Jusko WJ (2009) Scaling pharmacodynamics from in vitro and preclinical animal studies to humans. Drug Metab Pharmacokinet 24:16–24
Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C (2008) Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 7:1851–1863
Ng CM, Joshi A, Dedrick RL, Garovoy MR, Bauer RJ (2005) Pharmacokinetic-pharmacodynamic-efficacy analysis of efalizumab in patients with moderate to severe psoriasis. Pharm Res 22:1088–1100
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Core team (eds) (2009) nlme: linear and nonlinear mixed effects models. R package v. 3.1-93. http://cran.r-project.org/
Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, Folkes A, Gowan S, De Haven Brandon A, Di Stefano F, Hayes A, Henley AT, Lensun L, Pergl-Wilson G, Robson A, Saghir N, Zhyvoloup A, McDonald E, Sheldrake P, Shuttleworth S, Valenti M, Wan NC, Clarke PA, Workman P (2009) Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther 8:1725–1738
Rocchetti M, Del Bene F, Germani M, Fiorentini F, Poggesi I, Pesenti E, Magni P, De Nicolao G (2009) Testing additivity of anticancer agents in pre-clinical studies: a PK/PD modelling approach. Eur J Cancer 45:3336–3346
Salphati L, Wong H, Belvin M, Bradford D, Edgar KA, Prior WW, Sampath D, Wallin JJ (2010) Pharmacokinetic-pharmacodynamic modeling of tumor growth inhibition and biomarker modulation by the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos 38:1436–1442
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304:554
Sarker D, Reid AH, Yap TA, de Bono JS (2009) Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 15:4799–4805
Sausville EA, Burger AM (2006) Contributions of human tumor xenografts to anticancer drug development. Cancer Res 66: 3351–3354, discussion 3354
Schreck R, Rapp UR (2006) Raf kinases: Oncogenesis and drug discovery. Int J Cancer 119:2261–2271
Sebolt-Leopold JS, Herrera R (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4:937–947
Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M, Heynck S, Stuckrath I, Weiss J, Fischer F, Michel K, Goel A, Regales L, Politi KA, Perera S, Getlik M, Heukamp LC, Ansen S, Zander T, Beroukhim R, Kashkar H, Shokat KM, Sellers WR, Rauh D, Orr C, Hoeflich KP, Friedman L, Wong KK, Pao W, Thomas RK (2009) Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA 106:18351–18356
Staal SP (1987) Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA 84:5034–5037
Tanaka C, O’Reilly T, Kovarik JM, Shand N, Hazell K, Judson I, Raymond E, Zumstein-Mecker S, Stephan C, Boulay A, Hattenberger M, Thomas G, Lane HA (2008) Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol 26:1596–1602
Teicher BA (2006) Tumor models for efficacy determination. Mol Cancer Ther 5:2435–2443
Wang S, Guo P, Wang X, Zhou Q, Gallo JM (2008) Preclinical pharmacokinetic/pharmacodynamic models of gefitinib and the design of equivalent dosing regimens in EGFR wild-type and mutant tumor models. Mol Cancer Ther 7:407–417
Wang S, Zhou Q, Gallo JM (2009) Demonstration of the equivalent pharmacokinetic/pharmacodynamic dosing strategy in a multiple-dose study of gefitinib. Mol Cancer Ther 8:1438–1447
Wong H, Belvin M, Herter S, Hoeflich KP, Murray LJ, Wong L, Choo EF (2009) Pharmacodynamics of 2-[4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-3-(pyridine-4-yl)-1 H-pyrazol-1-yl]ethan-1-ol (GDC-0879), a potent and selective B-Raf kinase inhibitor: understanding relationships between systemic concentrations, phosphorylated mitogen-activated protein kinase kinase 1 inhibition, and efficacy. J Pharmacol Exp Ther 329:360–367
Wong H, Choo EF, Alicke B, Ding X, La H, McNamara E, Theil FP, Tibbitts J, Friedman LS, Hop CE, Gould SE (2012) Anti-tumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clin Cancer Res 18:3846–3855
Zebisch A, Troppmair J (2006) Back to the roots: the remarkable RAF oncogene story. Cell Mol Life Sci 63:1314–1330
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2012_1988_MOESM1_ESM.ppt
Representative individual tumor volume–time profiles and model prediction from animals that received a dose of vehicle (A) GDC-0973 6 mg/kg QD (B) GDC-0941 100 mg/kg QD (C) and combination of GDC-0973 6 mg/kg and GDC-0941 10 mg/kg QD (D). Observed tumor volumes (open circles); model-predicted tumor volume (solid line) (PPT 102 kb)
280_2012_1988_MOESM2_ESM.ppt
Representative comparisons of simulated versus. observed results from the xenograft studies. GDC-0973 10 mg/kg and GDC-0941 50 mg/kg Q3D (A). GDC-0973 10 mg/kg and GDC-0941 150 mg/kg Q3D (B); Observed data (solid circle and error bar; mean ± SD; n = 7); mean values of the simulated results (solid line); 5 and 95 % quantile values of simulated results; N = 1000 for the simulated results (dotted line) (PPT 150 kb)
Rights and permissions
About this article
Cite this article
Choo, E.F., Ng, C.M., Berry, L. et al. PK-PD modeling of combination efficacy effect from administration of the MEK inhibitor GDC-0973 and PI3K inhibitor GDC-0941 in A2058 xenografts. Cancer Chemother Pharmacol 71, 133–143 (2013). https://doi.org/10.1007/s00280-012-1988-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1988-6