Abstract
Purpose
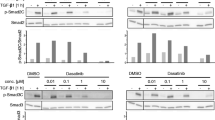
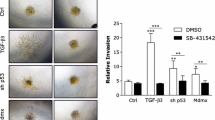
We have previously demonstrated that in pancreatic ductal adenocarcinoma (PDAC)-derived cell lines, the common Src family kinase inhibitors PP2 and PP1 effectively inhibited morphologic alterations associated with TGFβ1-mediated epithelial-to-mesenchymal transition (EMT) by blocking the kinase activity of the TGF-β type I receptor ALK5 rather than Src (Ungefroren et al. in Curr Cancer Drug Targets 11:524, 2011). In this report, the ability of PP2 and PP1, the more specific Src inhibitor SU6656, and the ALK5 inhibitor SB431542 to functionally block TGF-β1-dependent EMT and cell motility in established PDAC (Panc-1, Colo 357) and primary NSCLC (Tu459) cell lines were investigated.
Methods
The effects of PP2, PP1, SU6656, and SB431542 on TGF-β1-dependent cell scattering/EMT, cell migration/invasion, and expression of invasion-associated genes were measured by using the real-time cell analysis assay on the xCELLigence system and quantitative real-time RT-PCR, respectively.
Results
In all three cell lines tested, PP1, PP2, and SB431542 effectively blocked TGF-β1-induced cell scattering/EMT, migration, and invasion and in Colo 357 cells inhibited the induction of the invasion-associated MMP2 and MMP9 genes. In contrast, SU6656 only blocked TGF-β1-induced invasion in Panc-1 and Tu459 but not Colo 357 cells. PP1, and to a greater extent PP2, also inhibited the high spontaneous migratory activity of Panc-1 cells expressing a kinase-active ALK5 mutant.
Conclusions
These data provide evidence that PP2 and PP1 are powerful inhibitors of TGF-β-induced cell migration and invasion in vitro and directly target ALK5. Both agents may be useful as dual TGF-β/Src inhibitors in experimental therapeutics to prevent metastatic spread in late-stage PDAC and NSCLC.






Similar content being viewed by others
Abbreviations
- ALK5:
-
Activin receptor-like kinase 5
- DMSO:
-
Dimethyl sulfoxide
- EMT:
-
Epithelial-to-mesenchymal transition
- FCS:
-
Fetal calf serum
- NSCLC:
-
Non-small cell lung carcinoma
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PP1:
-
4-Amino-5-(4-methylphenyl)-7-(t-butyl) pyrazolo[3,4-d] pyrimidine
- PP2:
-
4-Amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d] pyrimidine
- PP3:
-
4-Amino-7-phenyl pyrazolo[3,4-d] pyrimidine
- siRNA:
-
Small interfering RNA
- SFK:
-
Src family kinase
- TBP:
-
TATA box-binding protein
- TGF-β:
-
Transforming growth factor-β
References
Gaspar NJ, Li L, Kapoun AM et al (2007) Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol 72:152–161
Zu X, Zhang Q, Cao R et al (2012) Transforming growth factor-β signaling in tumor initiation, progression and therapy in breast cancer: an update. Cell Tissue Res 347:73–84
Jeon HS, Jen J (2010) TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. J Thorac Oncol 5:417–419
Jones S, Zhang X, Parsons DW et al (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–1806
Calorini L, Bianchini F (2010) Environmental control of invasiveness and metastatic dissemination of tumor cells: the role of tumor cell-host cell interactions. Cell Commun Signal 8:24
Friess H, Yamanaka Y, Büchler M et al (1993) Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 105:1846–1856
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425:577–584
Schniewind B, Groth S, Sebens Müerköster S et al (2007) Dissecting the role of TGF-beta type I receptor/ALK5 in pancreatic ductal adenocarcinoma: smad activation is crucial for both the tumor suppressive and prometastatic function. Oncogene 26:4850–4862
Melisi D, Ishiyama S, Sclabas GM et al (2008) LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther 7:829–840
Wendt MK, Tian M, Schiemann WP (2012) Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res 347:85–101
Giampieri S, Pinner S, Sahai E (2010) Intravital imaging illuminates transforming growth factor beta signaling switches during metastasis. Cancer Res 70:3435–3439
Brábek J, Mierke CT, Rösel D et al (2010) The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commun Signal 8:22
Ellenrieder V, Hendler SF, Ruhland C et al (2001) TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer 93:204–211
Kim LC, Song L, Haura EB (2009) Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol 6:587–595
Nagaraj NS, Datta PK (2010) Targeting the transforming growth factor-β signaling pathway in human cancer. Expert Opin Investig Drugs 19:77–91
Maeda M, Shintani Y, Wheelock MJ, Johnson KR (2006) Src activation is not necessary for Transforming growth factor (TGF)-β-mediated epithelial to mesenchymal transitions (EMT) in mammary epithelial cells. PP1 directly inhibits TGF-beta receptors I and II. J Biol Chem 281:59–68
Ungefroren H, Sebens S, Groth S et al (2011) The Src family kinase inhibitors PP2 and PP1 block TGF-beta1-mediated cellular responses by direct and differential inhibition of type I and type II TGF-beta receptors. Curr Cancer Drug Targets 11:524–535
Medicherla S, Li L, Ma JY et al (2007) Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment. Anticancer Res 27:4149–4157
Keese CR, Bhawe K, Wegener J et al (2002) Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques 33:842–844, 846, 848–850
Atienza JM, Yu N, Kirstein SL et al (2006) Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol 4:597–607
Blake RA, Broome MA, Liu X et al (2000) SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20:9018–9027
Inman GJ, Nicolas FJ, Callahan JF et al (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74
Groth S, Schulze M, Kalthoff H et al (2005) Adhesion and Rac1-dependent regulation of biglycan gene expression by transforming growth factor-beta. Evidence for oxidative signaling through NADPH oxidase. J Biol Chem 280:33190–33199
Chen HC (2005) Cell-scatter assay. Methods Mol Biol 294:69–77
Wieser R, Wrana JL, Massagué J (1995) GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J 14:2199–2208
Ungefroren H, Sebens S, Groth S et al (2011) Differential roles of Src in transforming growth factor-ß regulation of growth arrest, epithelial-to-mesenchymal transition and cell migration in pancreatic ductal adenocarcinoma cells. Int J Oncol 38:797–805
Ito H, Gardner-Thorpe J, Zinner MJ et al (2003) Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery 134:221–226
Duxbury MS, Ito H, Zinner MJ et al (2004) Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res 10:2307–2318
Ischenko I, Camaj P, Seeliger H (2008) Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene 27:7212–7222
Acknowledgments
We thank H. Albrecht and S. Grammerstorf for their excellent technical assistance and Dr. J. Massagué (Memorial Sloan-Kettering Cancer Center, NY) for generously providing the ALK5T204D plasmid.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartscht, T., Lehnert, H., Gieseler, F. et al. The Src family kinase inhibitors PP2 and PP1 effectively block TGF-beta1-induced cell migration and invasion in both established and primary carcinoma cells. Cancer Chemother Pharmacol 70, 221–230 (2012). https://doi.org/10.1007/s00280-012-1904-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1904-0