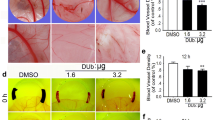
Abstract
Purpose
To assess the anti-angiogenesis potential and mechanism of RY10-4, a derivative of protoapigenone, which was verified the broad-spectrum anti-tumor activities by previous study.
Methods
RY10-4 and RY10-3 were synthesized according to the procedure described. Breast cancer cells MCF-7 and MDA-MB-231 that got the best performance in the previous anti-tumor activity screening were selected for further anti-cancer mechanism research. Firstly, cells proliferation assay of RY10-4 and RY10-3 was used to demonstrate the fact that the 4-hydroxy-2,5-cyclohexadien-1-one system would be the efficient pharmacophore of RY10-4. Then, a series of assays such as human umbilical vein endothelial cells (HUVECs) proliferation assay, HUVECs migration, tube network formation and morphological observations of zebrafish were applied to confirm its anti-angiogenesis activity. Upon RY10-4 treatment, the HIF-1α and VEGF were analyzed by western blot in normoxic and hypoxic conditions, meanwhile the PI3K-AKT-mTOR pathway-related protein such as AKT, p-AKT, mTOR and p-mTOR was also analyzed.
Results
In the MCF-7, MDA-MB-231 and HUVECs proliferation assay, RY10-4 that has 4-hydroxy-2,5-cyclohexadien-1-one system showed distinct advantage compared with RY10-3. Tests had verified the anti-angiogenesis capability of RY10-4. Down-regulation of the HIF-1α and inhibition phosphorylation levels of AKT and mTOR were found to be the pathway that RY10-4 exerts its functions on anti-angiogenesis.
Conclusions
The structure of 4-hydroxy-2,5-cyclohexadien-1-one should be the effective pharmacophore of RY10-4. RY10-4 got fine performance in anti-tumor and anti-angiogenesis assay, and thus, the quinol compound will be the new hot-spot for further anti-tumor agency development.






Similar content being viewed by others
References
Mohammad AR, Masakazu T (2003) Anti-angiogenic therapy in breast cancer. Biomed Pharmacother 57:463–470
Senn HJ (1982) Current status and indications for adjuvant therapy in breast cancer. Cancer Chemother Pharmacol 8:139–150
Susumu S, Hiromi J, Kenichi W, Masato T (2011) Does primary tumor resection improve outcomes for patients with incurable advanced breast cancer? Breast 20:543–547
Huang XH, Xiong PC, Xiong CM, Cai YL, Wei AH, Wang JP, Liang XF, Ruan JL (2010) In vitro and in vivo antitumor activity of Macrothelypteris torresiana and its acute/subacute oral toxicity. Phytomedicine 17:930–934
Yuan QY, Liu ZW, Xiong CM, Wu LQ, Wang JP, Ruan JL (2011) A novel, broad-spectrum antitumor compound containing the 1-hydroxycyclohexa-2, 5-dien-4-one group: the disclosure of a new antitumor pharmacophore in protoapigenone 1. Bioorg Med Chem Lett 21:3427–3430
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Yancopoulos GD, Klagsbrun M, Folkman J (1998) Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell 93:661–664
Folkman J (1990) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4–6
Parangi S, O’Reilly M, Christofori G et al (1996) Antiangiogenic therapy of transgenic mice impairs de novo tumor-growth. Proc Natl Acad Sci USA 93:2002–2007
Harris AL (1997) Antiangiogenesis for cancer therapy. Lancet 349(suppl II):13–15
Claffey KP, Robinson GS (1996) Regulation of VEGF/VPF expression in tumor cells: consequences for tumor-growth and metastasis. Cancer Metast Rev 15:165–176
Longo R, Gasparini G (2007) Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol 60:151–170
Pandit B, Sun YJ, Chen P, Sackett DL, Hu ZG, Rich W, Li PK et al (2006) Structure-activity-relationship studies of conformationally restricted analogs of combretastatin A-4 derived from SU5416. Bioorgan Med Chem 14:6492–6501
Xu G, Zhang WH, Betram P, Zheng XF, McLeod H (2004) Pharmacogenomic profiling of the PI3 K/PTEN-Akt-mTOR pathway in common human tumors. Int J Oncol 24:893–900
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Datta NS, Long MW (2002) Modulation of MDM2/p53 and cyclin-activating kinase during the megakaryocyte differentiation of human erythroleukemia cells. Exp Hematol 30:158–165
Taraboletti G, Giavazzi R (2004) Modelling approaches for angiogenesis. Eur J Cancer 40:881–889
Shin DH, Kim JH, Jung YJ, Kim KE, Jeong JM, Chun YS, Park JW (2007) Preclinical evaluation of YC-1, a HIF inhibitor, for the prevention of tumor spreading. Cancer Lett 255:107–116
Chuang TC, Hsu SC, Cheng YT, Shao WS, Wu KH, Fang GS, Ou CC, Wang V (2011) Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett 311:11–19
Lin AS, Kyoko NG, Chang FR, Yu DL, Lee KH et al (2007) First total synthesis of protoapigenone and its analogues as potent cytotoxic agents. J Med Chem 50:3921–3927
Chang HL, Wu YC, Su JH, Yeh YT, Yuan SF (2008) Protoapigenone, a novel flavonoid, induces apoptosis in human prostate cancer cells through activation of p38 mitogen-activated protein kinase and c-Jun NH2-Terminal kinase 1/2. J Pharmacol Exp Ther 325:841–849
Wei AH, Zhou DN, Xiong CM, Cai YL, Ruan JL (2011) A novel non-aromatic B-ring flavonoid: isolation, structure elucidation and its induction of apoptosis in human colon HT-29 tumor cell via the reactive oxygen species-mitochondrial dysfunction and MAPK activation. Food Chem Toxicol 49:2445–2452
Liu HB, Xiao YL, Xiong CM, Wei AH, Ruan JL (2008) Apoptosis induced by a new flavonoid in human hepatoma HepG2 cells involves reactive oxygen species-mediated mitochondrial dysfunction and MAPK activation. Eur J Pharmacol 654:209–216
Bradshaw TD, Matthews CS, Cookson J et al (2005) Elucidation of thioredoxin as a molecular target for antitumor quinols. Cancer Res 65:3911–3919
Chen WY, Hsieh YA, Tsai CI, Kang YF, Chang FR, Wu YC, Wu CC (2011) Protoapigenone, a natural derivative of apigenin, induces mitogen-activated protein kinase-dependent apoptosis in human breast cancer cells associated with induction of oxidative stress and inhibition of glutathione S-transferase π. Invest New Drug 29:1347–1359
Mayumi O, Hiroto I, Shigeo Y, Daisuke G, Michihiko K et al (1996) Angiogenesis as a new target for cancer treatment. Cancer Chemother Pharmacol 38(Suppl):S78–S82
Zhong XS, Zheng JZ, Reed E, Jiang BH (2004) SU5416 inhibited VEGF and HIF-1α expression through the PI3 K/AKT/p70S6K1 signaling pathway. Biochem Biophy Res Co 324:471–480
Comito G, Calvani M, Giannoni E, Bianchini F, Chiarugi P et al (2011) HIF-1α stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radic Biol Med 51:893–904
Manolescu B, Oprea E, Busu C, Cercasov C (2009) Natural compounds and the hypoxia-inducible factor (HIF) signalling pathway. Biochimie 91:1347–1358
Semenza GL (2000) Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol 35(2):71–103
Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Sawyers CL et al (2001) Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA 98:10314–10319
Chiang CT, Way TD, Tsai SJ, Lin JK (2007) Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett 581:5735–5742
Liu D, Xu YJ, Rao ZC, Chen ZC (2011) Preparation of anti-HER2 monoclonal antibody-paclitaxel immunoconjugate and its biological evaluation. J Huazhong Univ Sci Technol [Med Sci] 31(6):735–740
Chen HJ, Xiong T, Qu Y, Zhao FY, Ferriero D, Mu D (2012) mTOR activates hypoxia-inducible factor-1α and inhibits neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Neurosci Lett 507:118–123
Acknowledgments
This research was gratefully acknowledged by the grant (No. 30973864) from the National Nature Science Foundation of China, the grant (No. 2009CDA067) from the Nature Science Foundation of Hubei Province and the grant (No. 201260523182) from Wuhan Science and Technology Plan Foundation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Yuan, Q., Zhang, X. et al. RY10-4, a novel anti-tumor compound, exhibited its anti-angiogenesis activity by down-regulation of the HIF-1α and inhibition phosphorylation of AKT and mTOR. Cancer Chemother Pharmacol 69, 1633–1640 (2012). https://doi.org/10.1007/s00280-012-1873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1873-3