Abstract
Purpose
Chidamide (CS055/HBI-8000) is a new benzamide class of histone deacetylase inhibitor with marked anti-tumor activity. This study reports the phase I results.
Methods
Patients with advanced solid tumors or lymphomas received oral doses of 5, 10, 17.5, 25, 32.5, or 50 mg chidamide either twice (BIW) or three times (TIW) per week for 4 consecutive weeks every 6 weeks. Safety, characteristics of pharmacokinetics (PK) and pharmacodynamics (PD), and preliminary efficacy were evaluated.
Results
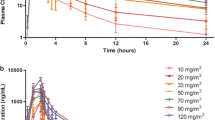
A total of 31 patients were enrolled. No DLTs were identified in the BIW cohorts up to 50 mg. DLTs were grade 3 diarrhea and vomiting in two patients in the TIW cohort at 50 mg, respectively. PK analysis revealed t1/2 of 16.8–18.3 h, T max of 1–2 h in most cases, and a dose-related increase in C max and AUC. Significant induction of histone H3 acetylation in peripheral white blood cells was observed after a single dose of chidamide. Four patients with T-cell lymphomas and 1 patient with submandibular adenoid cystic carcinoma achieved a partial response.
Conclusions
Chidamide was generally well tolerated in patients with advanced solid tumors or lymphomas in the tested regimens. Favorable PK and PD profiles, as well as encouraging preliminary anti-tumor activity, were demonstrated.


Similar content being viewed by others
References
Mai A, Altucci L (2009) Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol 41:199–213
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10:32–42
Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6:38–51
Rasheed W, Bishton M, Johnstone RW, Prince HM (2008) Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev Anticancer Ther 8:413–432
Nakagawa M, Oda Y, Eguchi T, Aishima S, Yao T, Hosoi F, Basaki Y, Ono M, Kuwano M, Tanaka M, Tsuneyoshi M (2007) Expression profile of class I histone deacetylases in human cancer tissues. Oncol Rep 18:769–774
Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G (2008) Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer 98:604–610
Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, Bhalla K (2006) A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 12:4628–4635
Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Duvic M (2007) Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25:3109–3115
Crump M, Andreadis C, Assouline S, Rizzieri D, Wedgwood A, McLaughlin P, Laille E, Li Z, Martell RE, Younes A (2008) Treatment of relapsed or refractory non-Hodgkin lymphoma with the oral isotype-selective histone deacetylase inhibitor MGCD0103: interim results from a Phase II study. J Clin Oncol 26 (Abstract 8528)
Gimsing P, Hansen M, Knudsen LM, Knoblauch P, Christensen IJ, Ooi CE, Buhl-Jensen P (2008) A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol 81:170–176
FDA (2006) US Food and Drug Administration, Center for Drug Evaluation and Research, Office of Oncology Drug Products (OODP). Approval letter for ZOLINZA™, NDA 21-991, Merck & Co. Inc
FDA (2009) US Food and Drug Administration, Center for Drug Evaluation and Research, Office of Oncology Drug Products (OODP). Approval letter for ISTODAX, NDA 022393, Gloucester Pharmaceuticals
FDA (2011) US Food and Drug Administration, Center for Drug Evaluation and Research, Office of Oncology Drug Products (OODP). Approval letter for ISTODAX, NDA 022393/S-004, Celgene Corporation
Blumenschein GR Jr, Kies MS, Papadimitrakopoulou VA, Lu C, Kumar AJ, Ricker JL, Chiao JH, Chen C, Frankel SR (2008) Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs 26:81–87
Modesitt SC, Sill M, Hoffman JS, Bender DP, Gynecologic Oncology Group (2008) A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 109:182–186
Vansteenkiste J, Van Cutsem E, Dumez H, Chen C, Ricker JL, Randolph SS, Schoffski P (2008) Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or non-small cell lung cancer. Invest New Drugs 26:483–488
Ramalingam SS, Parise RA, Ramanathan RK, Lagattuta TF, Musguire LA, Stoller RG, Potter DM, Argiris AE, Zwiebel JA, Egorin MJ, Belani CP (2007) Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res 13:3605–3610
Munster PN, Lacevic M, Thomas S, Christian R, Ismail-Khan R, Melisko M, Rugo H, Minton SE (2009) Phase II trial of the histone deacetylase inhibitor, vorinostat, to restore hormone sensitivity to the antiestrogen tamoxifen in patients with advanced breast cancer who progressed on prior hormone therapy. J Clin Oncol 27(Suppl) (Abstract 1075)
Ramalingam SS, Maitland M, Frankel P, Argiris AE, Koczywas M, Gitlitz B, Espinoza-Delgado I, Vokes EE, Gandara DR, Belani CP (2009) Randomized, double-blind, placebo-controlled Phase II study of carboplatin and paclitaxel with or without vorinostat, a histone deacetylase inhibitor (HDAC), for first-line therapy of advanced non-small cell lung cancer (NCI 7863). J Clin Oncol 27 (Suppl) (Abstract 8004)
Nolan L, Johnson PW, Ganesan A, Packham G, Crabb SJ (2008) Will histone deacetylase inhibitors require combination with other agents to fulfil their therapeutic potential? Br J Cancer 99:689–694
Xie AH, Liao CZ, Li ZB, Ning ZQ, Hu W, Lu XP, Shi LM, Zhou J (2004) Quantitative structure-activity relationship study of histone deacetylase inhibitors. Curr Med Chem Anticancer Agents 4:273–299
Yin ZH, Wu ZW, Lan YK, Liao CZ, Shan S, Li ZL, Ning ZQ, Lu XP, Li ZB (2004) Synthesis of chidamide, a new histone deacetylase (HDAC) inhibitor. Chin J New Drugs 13:536–538
Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP (2011) Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharm. doi: 10.1007/s00280-011-1766-x
Glaser KB (2007) HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol 74:659–671
Rasheed W, Bishton M, Johnstone RW, Prince HM (2008) Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev Anticancer Ther 8:413–432
Kummar S, Gutierrez M, Gardner ER, Donovan E, Hwang K, Chung EJ, Lee MJ, Maynard K, Kalnitskiy M, Chen A, Melillo G, Ryan QC, Conley B, Figg WD, Trepel JB, Zwiebel J, Doroshow JH, Murgo AJ (2007) Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res 13:5411–5417
Gore L, Rothenberg ML, O’Bryant CL, Schultz MK, Sandler AB, Coffin D, McCoy C, Schott A, Scholz C, Eckhardt SG (2008) A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin Cancer Res 14:4517–4525
Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M (2008) Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 409:581–589
Bonfils C, Kalita A, Dubay M, Siu LL, Carducci MA, Reid G, Martell RE, Besterman JM, Li Z (2008) Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clin Cancer Res 14:3441–3449
Chou CJ, Herman D, Gottesfeld JM (2008) Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases. J Biol Chem 283:35402–35409
Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Duvic M (2007) Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25:3109–3115
Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, Scarisbrick J, Reddy S, Robak T, Becker JC, Samtsov A, McCulloch W, Kim YH (2010) Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 28:4485–4491
Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N (2005) Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res 65:11136–11145
Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, Steinle A, Salih HR (2005) Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 65:6321–6329
Acknowledgments
The authors thank Xiao-Rong Liu, Song Shan, and Jing-Zhong Zhu from Chipscreen Biosciences for analysis of histone acetylation. We also thank Dr. Michael J. Newman from HUYA Bioscience International for the critical review of the manuscript. This work was partially supported by grants from the Chinese National “863” Project (2008AA02Z303), National Prize for Small- and Middle-sized enterprises (04C26214420752), and the Significant Project in Biotech Field from Guangdong Province (2003A10903) and Shenzhen City (2005-K2-009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, M., Ning, ZQ., Xing, PY. et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol 69, 1413–1422 (2012). https://doi.org/10.1007/s00280-012-1847-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1847-5