Abstract
Purpose
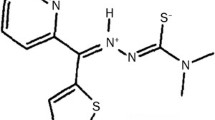
Preclinical studies evaluated the anti-tumor activity and mechanism of action of AMP423, a naphthyl derivative of 2-cyanoaziridine-1-carboxamide with structural similarity to the pro-oxidant anti-tumor agent imexon.
Methods
The cytotoxic potency was evaluated in vitro against a variety of human cancer cell lines. Mechanism-of-action studies were performed in the human 8226/S myeloma cell line and its imexon-resistant variant, 8226/IM10. In vivo activity was evaluated against human myeloma and lymphoma xenografts in SCID mice. Pharmacokinetics and toxicology were investigated in non-tumor-bearing mice.
Results
The 72-h IC50s for all cell types ranged from 2 to 36 μM, across a wide variety of human cancer cell lines. AMP423 was active in SCID mice bearing 8226/S myeloma and SU-DHL-6 B-cell lymphoma tumors, with a median tumor growth delay (T−C) of 21 days (P = 0.0002) and 5 days (P = 0.004), respectively, and a median tumor growth inhibition (T/C) of 33.3% (P = 0.03) and 82% (P = 0.01), respectively. In non-tumor-bearing mice, AMP423 was not myelosuppressive. Mechanistic studies show that AMP423’s mode of cell death is a mixture of necrosis and apoptosis, with generation of reactive oxygen species, inhibition of protein synthesis, and a decrease in reduced sulfhydryl levels, but no alkylation of nucleophiles. Unlike its structural analog imexon, which causes cell cycle arrest in G2/M, AMP423 induces the accumulation of cells in S-phase.
Conclusions
AMP423 has pro-oxidant effects similar to imexon, has greater cytotoxic potency in vitro, and has anti-tumor activity in hematologic tumors in vivo.







Similar content being viewed by others
References
Iyengar BS, Dorr RT, Alberts DS, Hersh EM, Salmon SE, Remers WA (1999) Novel antitumor 2-cyanoaziridine-1-carboxamides. J Med Chem 42:510–514
Salmon SE, Hersh EM (1994) Sensitivity of multiple myeloma to imexon in the human tumor cloning assay. J Natl Cancer Inst 86:228–230
Hersh EM, Gschwind CR, Taylor CW, Dorr RT, Taetle R, Salmon SE (1992) Antiproliferative and antitumor activity of the 2-cyanoaziridine compound imexon on tumor cell lines and fresh tumor cells in vitro. J Natl Cancer Inst 84:1238–1244
Dorr RT, Liddil JD, Klein MK, Hersh EM (1995) Preclinical pharmacokinetics and antitumor activity of imexon. Invest New Drugs 13:113–116
Pourpak A, Meyers RO, Samulitis BK, Chow H–H, Kelper CY, Raymond MA, Hersh E, Dorr RT (2006) Preclinical antitumor activity, pharmacokinetics and pharmacodynamics of imexon in mice. Anticancer Drugs 17(10):1179–1184
Iyengar BS, Dorr RT, Remers WA (2004) Chemical basis for the biological activity of imexon and related cyanoaziridines. J Med Chem 47:218–223
Dvorakova K, Payne CM, Tome M, Briehl MM, McClure T, Dorr RT (2000) Induction of oxidative stress and apoptosis in myeloma cells the aziridine-containing agent by imexon. Biochem Pharmacol 60:749–758
Samulitis BK, Landowski TH, Dorr RT (2006) Correlates of imexon sensitivity in human multiple myeloma cell lines. Leuk Lymphoma 47:97–109
Dvorakova K, Waltmire CN, Payne CM, Tome ME, Briehl MM, Dorr RT (2001) Induction of mitochondrial changes in myeloma cells by imexon. Blood 97:3544–3551
Dvorakova K, Payne CM, Landowski TH, Tome ME, Halperin DS, Dorr RT (2002) Imexon activates an intrinsic apoptosis pathway in RPMI8226 myeloma cells. Anticancer Drugs 13:1031–1042
Dorr RT, Raymond MA, Landowski TH, Roman NO, Fukushima S (2005) Induction of apoptosis and cell cycle arrest by imexon in human pancreatic cancer cell lines. Int J Gastrointest Cancer 36:15–28
Dragovich T, Gordon M, Mendelson D, Wong L, Modiano M, Chow S, Samulitis B, O’Day S, Grenier K, Hersh E, Dorr RT (2007) Phase I trial of imexon in patients with advanced malignancy. J Clin Oncol 25:1779–1784
Weber JS, Samlowski WE, Gonzalez R, Ribas A, Stephenson J, O’Day S, Sato T, Dorr R, Grenier K, Hersh E (2010) A phase I-II study of imexon plus dacarbazine (DTIC) in patients with unresectable metastatic melanoma. Cancer 116(15):3683–3691
Moulder S, Dhillon N, Ng C, Hong D, Wheler J, Naing A, Tse S, La Paglia A, Dorr R, Hersh E, Boytim M, Kurzrock A (2010) A phase I trial of imexon, a pro-oxidant, in combination with docetaxel for the treatment of patients with advanced breast, non-small cell lung and prostate cancer. Invest New Drugs 29(5):634–640
Cohen SJ, Zalupski MM, Modiano MR, Conkling PR, Patt YZ, Davis P, Dorr RT, Boytim ML, Hersh EM (2010) A phase I study of imexon plus gemcitabine as first-line therapy for advanced pancreatic cancer. Cancer Chemother Pharmacol 66(2):287–294
Scott J, Dorr RT, Samulitis B, Landowski TH (2007) Imexon-based combination chemotherapy in A375 human melanoma and RPMI 8226 human myeloma cells lines. Cancer Chemother Pharmacol 59:749–757
Dvorakova K, Payne CM, Tome ME, Briehl MM, Vasquez MA, Waltmire CR, Coon A, Dorr RT (2002) Molecular and cellular characterization of imexon-resistant RPMI 8226/I myeloma cells. Mol Cancer Ther 1:185–195
Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE (1986) Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Res 46:5125–5130
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Reers M, Smith TW, Chen LB (1991) J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 30(18):4480–4486
Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B (2003) Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34:1359–1368
Poot M, Kavanagh TJ, Kang HC, Haugland RP, Rabinovitch PS (1991) Flow cytometric analysis of cell cycle-dependent changes in cell thiol level by combining a new laser dye with Hoechst 333421. Cytometry 12:184–187
Friedman DM, Boger E (1961) Colorimetric estimation of nitrogen mustards in aqueous media. Anal Chem 33:906–910
Christian RA, Chaffee SK, Hovick CJ, Steele WJ (1980) A stable colorimetric assay for cyclophosphamide and its alkylating metabolites based on the alkylation of 4-(4′-nitrobenzyl)-pyridine. Life Sci 27:2595–2599
Bissery MC, Guenard D, Cueritte-Voegelein F, Lavelle F (1991) Experimental antitumor activity of Taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res 51:4845–4852
Samulitis BK, Landowski TH, Dorr RT (2009) Inhibition of protein synthesis by imexon reduces HIF-1alpha expression in normoxic and hypoxic pancreatic cancer cells. Invest New Drugs 27:89–98
Tomasz M, Chowdary D, Lipman R, Shimotakahara S, Veiro D, Walker V, Verdine GL (1986) Reaction of DNA with chemically or enzymatically activated mitomycin C: isolation and structure of the major covalent adduct. Proc Natl Acad Sci 83:6702–6706
Ismail FM, Levitsky DO, Dembitsky VM (2009) Aziridine alkaloids as potential therapeutic agents. Eur J Med Chem 44:3373–3387
Paleo MR, Aurrecoechea N, Jung K-Y, Rapoport H (2003) Formal enantiospecific synthesis of (+)−FR900482. J Org Chem 68:130–138
Tomasz M, Palom Y (1997) The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol Ther 76:73–87
Acknowledgments
This study is supported in part by grants CA-017094, CA-115626, and CA-023074 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, U.S.A. We thank the University of Arizona Chemical Synthesis Facility, and the University of Arizona Cancer Center Flow Cytometry Core Service, Experimental Mouse Shared Service, and the Biostatistical Core Service for their expertise.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2011_1784_MOESM1_ESM.tif
Analysis of binding to cellular thiols by AMP423 and imexon in 8226/S and 8226/IM10 cells. Binding to cellular thiols was analyzed by concurrent CMFDA and PI staining after 48 h exposure to increasing concentrations of AMP423 or imexon. Non-viable cells, indicated by positive PI staining, were gated out and the remaining population of viable cells were analyzed for intracellular reduced thiol content. The percent of CMFDA positive, viable cells is shown. Results shown are mean ± SEM (n = 3) (TIF 148 kb)
280_2011_1784_MOESM2_ESM.tif
Analysis of reactive oxygen species (ROS) upon exposure to AMP423 or imexon in 8226/S and 8226/IM10 cells. The increase in reactive oxygen species seen in 8226/S cells (Figs A, B) or 8226/IM10 cells (Figs C, D) after 24 h (left) or 48 h (right). The percent of HE positive cells is shown. Results shown are mean ± SEM (n = 6) (TIFF 166 kb)
Rights and permissions
About this article
Cite this article
Dorr, R.T., Wisner, L., Samulitis, B.K. et al. Anti-tumor activity and mechanism of action for a cyanoaziridine-derivative, AMP423. Cancer Chemother Pharmacol 69, 1039–1049 (2012). https://doi.org/10.1007/s00280-011-1784-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1784-8