Abstract
Purpose
The aim of this study was to evaluate a phenotypic cell panel with tumor cells from various patients and normal cells for preclinical profiles of antitumor efficacy and toxicity of anticancer drugs.
Methods
The antitumor activity of fourteen anticancer drugs was tested in over one hundred tumor samples from patients with solid or hematological malignancies. Drug activity against four normal cell types was used for the assessment of normal tissue toxicity. In vitro activity of the drugs was compared with indications approved by the Food and Drug Administration and established adverse event profiles.
Results
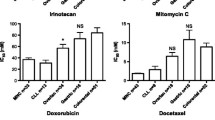
In general, in vitro drug activity in tumor cells from patients reflected known clinical activity of the drugs investigated. For example, the clinical activity of imatinib in chronic myeloid leukemia was clearly detected in the tumor panel. Further, and in accordance with clinical use, cisplatin and bortezomib showed high activity in ovarian cancer and myeloma samples, respectively. The normal cell models roughly reflected known clinical toxicity profiles and were able to detect differences in therapeutic index, e.g., between targeted drugs and classical cytotoxic agents. For example, the high tolerability of imatinib and the well-known renal toxicity of cisplatin were demonstrated.
Conclusions
In preclinical drug development, primary tumor cells from patients can be used for the prediction of cancer diagnosis–specific activity and may aid in the selection of diagnoses for clinical trials. By using tumor and toxicity panels together, information about therapeutic index may be derived, which may be useful when choosing among drug candidates with similar tumor effects.




Similar content being viewed by others
References
Damia G, D’Incalci M (2009) Contemporary pre-clinical development of anticancer agents–what are the optimal preclinical models? Eur J Cancer 45:2768–2781
Voskoglou-Nomikos T, Pater JL, Seymour L (2003) Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res 9:4227–4239
Peterson JK, Houghton PJ (2004) Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer 40:837–844
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM et al (2001) Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 84:1424–1431
Sharma SV, Haber DA, Settleman J (2010) Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nature reviews 10:241–253
Li W, Lam M, Choy D, Birkeland A, Sullivan ME et al (2006) Human primary renal cells as a model for toxicity assessment of chemo-therapeutic drugs. Toxicol In Vitro 20:669–676
Li AP, Bode C, Sakai Y (2004) A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem Biol Interact 150:129–136
Bosanquet AG, Bell PB (2004) Ex vivo therapeutic index by drug sensitivity assay using fresh human normal and tumor cells. J Exp Ther Oncol 4:145–154
Li W, Lam MS, Birkeland A, Riffel A, Montana L et al (2006) Cell-based assays for profiling activity and safety properties of cancer drugs. J Pharmacol Toxicol Methods 54:313–319
Fridborg H, Jonsson E, Nygren P, Larsson R (1999) Relationship between diagnosis-specific activity of cytotoxic drugs in fresh human tumour cells ex vivo and in the clinic. Eur J Cancer 35:424–432
Lindhagen E, Nygren P, Larsson R (2008) The fluorometric microculture cytotoxicity assay. Nat Protoc 3:1364–1369
Haglund C, Aleskog A, Hakansson LD, Hoglund M, Jacobsson S et al (2010) The FMCA-GM assays, high throughput non-clonogenic alternatives to CFU-GM in preclinical hematotoxicity testing. Toxicol Lett 194:102–107
Larsson R, Kristensen J, Sandberg C, Nygren P (1992) Laboratory determination of chemotherapeutic drug resistance in tumor cells from patients with leukemia, using a fluorometric microculture cytotoxicity assay (FMCA). Int J Cancer 50:177–185
FDA (2010) http://dailymed.nlm.nih.gov/dailymed/about.cfm. Food and Drug Administration. Available via Food and Drug Administration http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed October 2010
Ramström H (ed) (2009) Läkemedelsboken. 17 edn. Apoteket AB, Stockholm
FASS (ed) (2009) FASS. The Swedish Drug Compendium, Läkemedelsindustriförenningen AB
Kintzel PE (2001) Anticancer drug-induced kidney disorders. Drug Saf 24:19–38
Wolf D, Rumpold H (2009) A benefit-risk assessment of imatinib in chronic myeloid leukaemia and gastrointestinal stromal tumours. Drug Saf 32:1001–1015
Sanz M, Burnett A, Lo-Coco F, Lowenberg B (2009) FLT3 inhibition as a targeted therapy for acute myeloid leukemia. Curr Opin Oncol 21:594–600
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R et al (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372:1809–1818
Lindhagen E, Eriksson A, Wickstrom M, Danielsson K, Grundmark B et al (2008) Significant cytotoxic activity in vitro of the EGFR tyrosine kinase inhibitor gefitinib in acute myeloblastic leukaemia. Eur J Haematol 81:344–353
Boehrer S, Ades L, Galluzzi L, Tajeddine N, Tailler M et al (2008) Erlotinib and gefitinib for the treatment of myelodysplastic syndrome and acute myeloid leukemia: a preclinical comparison. Biochem Pharmacol 76:1417–1425
Stegmaier K, Corsello SM, Ross KN, Wong JS, Deangelo DJ et al (2005) Gefitinib induces myeloid differentiation of acute myeloid leukemia. Blood 106:2841–2848
Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K et al (2009) Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell 16:281–294
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E et al (2005) Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 105:54–60
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281
Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M et al (2009) Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 27:3822–3829
EMEA EMA Document Number EMA/783677/2009. Accessed 2010/09/30 2010
Holbeck SL, Collins JM, Doroshow JH (2010) Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol Cancer Ther 9:1451–1460
Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ et al (2005) Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood 106:3777–3784
Pessina A, Albella B, Bayo M, Bueren J, Brantom P et al (2003) Application of the CFU-GM assay to predict acute drug-induced neutropenia: an international blind trial to validate a prediction model for the maximum tolerated dose (MTD) of myelosuppressive xenobiotics. Toxicol Sci 75:355–367
Acknowledgments
The skillful technical assistance of Anna-Karin Lannergård, Christina Leek, Lena Lenhammar, David Munro and Linda Rickardson is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haglund, C., Åleskog, A., Nygren, P. et al. In vitro evaluation of clinical activity and toxicity of anticancer drugs using tumor cells from patients and cells representing normal tissues. Cancer Chemother Pharmacol 69, 697–707 (2012). https://doi.org/10.1007/s00280-011-1746-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1746-1