Abstract
Purpose
The epothilones are a novel class of microtubule-stabilizing agents. UTD1 is an epothilone analog generated by genetic manipulation of the polyketide biosynthetic gene cluster. This phase I study was designed to evaluate the safety and pharmacokinetic(PK) profiles of UTD1 in patients with advanced solid tumors.
Patients and methods
This was an open-label, single-arm, one site, phase I, dose-escalation study. Patients were treated with escalating doses of UTD1 as a 3-h intravenous infusion every 3 weeks.
Results
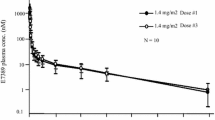
Twenty-one patients were enrolled and received UTD1 at six dose levels ranging from 25 to 225 mg/m2. Dose-limiting toxicity (DLT) was ataxia, and other frequent non-haematological toxicities were peripheral neuropathy, gastrointestinal disorders, fatigue, and myalgia/arthralgia. Myelosuppression was rare, with no grade 3 and 4 neutropenia, in contrast to paclitaxel and ixabepilone. The maximum-tolerated dose was established as 170 mg/m2. Preliminary results showed linear pharmacokinetics along the range of doses tested. Prolonged disease stabilization was observed in patients with breast cancer, non-small lung cancer, and other cancers.
Conclusions
The recommended phase II dose of UTD1 is 170 mg/m2 as a 3-h infusion every 3 weeks. Ataxia was the DLT. UTD1 showed advantages over paclitaxel and Ixapebilone in relation to safety profile, especially myelosuppression. The acceptable tolerability warrants further phase II study.


Similar content being viewed by others
References
Horwitz SB (1992) Mechanism of action of taxol. Trends Pharmacol Sci 13(4):134–136
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN (2007) Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol 608:1–22
Xu BH (2008) Epothilones in the treatment of breast cancer: review of clinical experience. Asia-Pac J Clin Oncol 4(Suppl. 3):S30–S39
Fumoleau P, Coudert B, Isambert N et al (2007) Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol 18(Suppl. 5):v9–v15
Goodin S, Kane MP, Rubin EH (2004) Epothilones: mechanism of action and biologic activity. J Clin Oncol 22(10):2015–2025
Thomas ES, Gomez HL, Li RK et al (2007) Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25(33):5210–5217
Lee JJ, Swain SM (2008) The epothilones: translating from the laboratory to the clinic. Clin Cancer Res 14(6):1618–1624
Altmann KH, Memmert K (2008) Epothilones as lead structures for new anticancer drugs–pharmacology, fermentation, and structure-activity-relationships. Prog Drug Res 66:275–334
Larkin JM, Kaye SB (2007) Potential clinical applications of epothilones: a review of phase II studies. Ann Oncol Suppl 5:v28–v34
Fornier MN (2007) Epothilones in breast cancer: review of clinical experience. Ann Oncol Suppl 5:v16–v21
Dawson NA (2007) Epothilones in prostate cancer: review of clinical experience. Ann Oncol Suppl 5:v22–v27
Puhalla S, Brufsky A (2008) Ixabepilone: a new chemotherapeutic option for refractory metastatic breast cancer. Biologics 2(3):505–515
Fornier MN (2007) Ixabepilone, first in a new class of antineoplastic agents: the natural epothilones and their analogues. Clin Breast Cancer 7(10):757–763
Ho J, Zhang L, Todorova L et al (2009) Budget impact analysis of ixabepilone used according to FDA approved labeling in treatment-resistant metastatic breast cancer. J Manag Care Pharm 15(6):467–475
Weiss RB, Donehower RC, Wiernik PH et al (1990) Hypersensitivity reactions from taxol. J Clin Oncol 8(7):1263–1268
Finley RS, Rowinsky EK (1994) Patient care issues: the management of paclitaxel-related toxicities. Ann Pharmacother 28(5 Suppl):S27–S30
Fossella FV, DeVore R, Kerr RN et al (2000) Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 non-small cell lung cancer study group. J Clin Oncol 18(12):2354–2362
Schmid P, Kiewe P, Possinger K et al (2010) Phase I study of the novel, fully synthetic epothilone sagopilone (ZK-EPO) in patients with solid tumors. Ann Oncol 21(3):633–639
Arnold D, Voigt W, Kiewe P et al (2009) BWeekly administration of sagopilone (ZK-EPO), a fully synthetic epothilone, in patients with refractory solid tumours: results of a phase I trial. Br J Cancer 101(8):1241–1247
Aghajanian C, Burris HA 3rd, Jones S et al (2007) Phase I study of the novel epothilone analog ixabepilone (BMS-247550) in patients with advanced solid tumors and lymphomas. J Clin Oncol 25(9):1082–1088
Shimizu T, Yamamoto N, Yamada Y et al (2008) ) Phase I clinical and pharmacokinetic study of 3-weekly, 3-h infusion of ixabepilone (BMS-247550), an epothilone B analog, in Japanese patients with refractory solid tumors. Cancer Chemother Pharmacol 61(5):751–758
Acknowledgments
We thank the patients for their participation and the study coordinators, nurses, physicians, and laboratory technicians for their assistance. This study was partly supported by a State Innovation Fund for Small and Medium Enterprise of China to R. Qiu and National HighTechnology Project Grant (2004AA2Z3T63) to B. Xu.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pin Zhang and Mingyuan Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, P., Sun, M., Qiu, R. et al. Phase I clinical and pharmacokinetic study of UTD1, a genetically engineered epothilone analog in patients with advanced solid tumors. Cancer Chemother Pharmacol 68, 971–978 (2011). https://doi.org/10.1007/s00280-011-1571-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1571-6