Abstract
Purpose
Standardized enumeration of CEC counts is required to minimize variability and allow cross-studies comparisons. The purpose of this paper is to identify CEC threshold proposal, by CellSearch system, for determining response to bevacizumab-based chemotherapy in metastatic colorectal cancer.
Methods
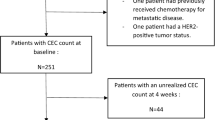
From July 2007 to June 2008, 33 patients treated with FOLFOX4 plus bevacizumab were enrolled in a prospective study. From January 2007 to June 2007, before bevacizumab was approved by the government in Japan, 31 patients treated with FOLFOX4 as a control were enrolled. CECs of whole blood at the baseline, day 4, 2 weeks after initiation of chemotherapy were isolated and counted using CellSearch system.
Results
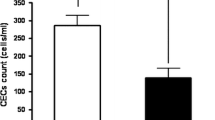
There was no correlation between CEC levels and the outcome in the FOLFOX4. In the bevacizumab-based chemotherapy, CEC levels at the baseline were significantly associated with the outcome. Patients with 65 or more CECs at the baseline had a shorter median PFS and OS, than the median PFS and OS of less than 65 CECs at the baseline in the bevacizumab-based chemotherapy (P = 0.003, P = 0.027, respectively). By univariate and multivariate Cox proportional-hazards regression, CEC levels (cut-off; 65) at the baseline indicated the strongest predictor for the outcome to bevacizumab-based chemotherapy.
Conclusion
A threshold of lower than 65 CECs, by the CellSearch System, at the baseline was a significant predictor of the outcome for colorectal cancer patients treated with bevacizumab-based chemotherapy.



Similar content being viewed by others
References
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern cooperative oncology group study E3200. J Clin Oncol 25:1539–1544
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334
Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cells in cancer: towards marker and target identification. Nat Rev Cancer 6:835–845
Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F (2001) Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97:3658–3661
Dellapasqua S, Bertolini F, Bagnardi V et al (2008) Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 26:4899–4905
Mancuso P, Colleni M, Calleri A et al (2006) Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 108:452–459
Torrisi R, Bagnardi V, Cardillo A et al (2008) Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER- and/or PgR-positive breast cancer: clinical and biological activity. Br J Cancer 99:1564–1571
Willett CG, Duda DG, di Tomaso E et al (2009) Efficacy, safety, biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 27:3020–3026
Duda DG, Cohen KS, di Tomaso E, Au P, Klein RJ, Scadden DT, Willett CG, Jain RK (2006) Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for antiangiogenic therapy. J Clin Oncol 24:1449–1453
Mancuso P, Antoniotti P, Quarna J et al (2009) Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res 15:267–273
Duda DG, Cohen KS, Scadden DT, Jain RK (2007) A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc 2:805–810
Rowand JL, Martin G, Doyle GV, Miller MC, Pierce MS, Connelly MC, Rao C, Terstappen LW (2007) Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A 71:105–113
Bidard FC, Mathiot C, Degeorges A et al (2010) Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 21:1765–1771
Calleri A, Bono A, Bagnardi V et al (2009) Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res 15:7652–7657
Ronzoni M, Manzoni M, Mariucci S, et al. (2010) Circulating endothelial cells and endothelia progenitors as predictive markers of clinical response to bevacizumab-based first-line treatment in advanced colorectal cancer patients. Ann Oncol May 23 [Epub ahead of print]
Acknowledgments
This work was supported by AstraZeneca Research Grant 2007, the Kobayashi Institute for Innovative Cancer Chemotherapy, and a Grant-in-Aid for Scientific Research (Japan Society for the Promotion of Science) (grant number 19790963, 21591741, 17016077). The excellent technical assistances of Sayuri Minowa, Harumi Shibata, and Mariko Kimura are greatly appreciated.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsusaka, S., Suenaga, M., Mishima, Y. et al. Circulating endothelial cells predict for response to bevacizumab-based chemotherapy in metastatic colorectal cancer. Cancer Chemother Pharmacol 68, 763–768 (2011). https://doi.org/10.1007/s00280-010-1543-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1543-2