Abstract
Purpose
To define the maximum-tolerated dose (MTD) for weekly paclitaxel administered in combination with daily vatalanib (PTK787/ZK 222584, PTK/ZK) and assess for a drug–drug interaction.
Methods
Patients were treated with escalating doses of weekly paclitaxel (75–85 mg/m2), and daily PTK/ZK (250–1,000 mg). During the first cycle only, paclitaxel was given on days 1 and 15, and PTK/ZK on days 3–28. Pharmacokinetic studies were conducted on cycle 1 days 1 and 15 for paclitaxel, and on cycle 1 day 15 for PTK/ZK. Therapy was given until disease progression.
Results
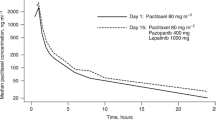
Twenty-seven patients were accrued to four dose levels. Two of five patients treated with paclitaxel 85 mg/m2 and PTK/ZK 1,000 mg had Grade 3 transaminase elevation as dose-limiting toxicity. Paired PK analyses demonstrated a significant increase in paclitaxel clearance on day 15 (p = 0.006). Activity included one partial response and 11 patients with stable disease ≥4 months, including patients previously treated with paclitaxel.
Conclusions
The MTD for weekly paclitaxel plus daily PTK/ZK is 75 mg/m2 and 750 mg. PK analysis revealed a significant drug–drug interaction, with an increase in paclitaxel clearance. This combination was well tolerated with evidence of anti-cancer activity and provides guidance for phase 2 planning.

Similar content being viewed by others
References
Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J (2000) PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60:2178–2189
Drevs J, Muller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Henniq J, Unger C, Marme D (2002) PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res 62:4015–4022
Lau DH, Xue L, Young LJ, Burke PA, Cheung AT (1999) Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm 14:31–36
Seidman AD, Hudis CA, Albanell J, Tonq W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L (1998) Dose-dense therapy with weekly 1-h paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353–3361
Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60:1878–1886
Kakeji Y, Maehara Y, Ikebe M, Teicher BA (1997) Dynamics of tumor oxygenation, CD31 staining and transforming growth factor-beta levels after treatment with radiation or cyclophosphamide in the rat 13762 mammary carcinoma. Int J Radiat Oncol Biol Phys 37:1115–1123
Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW Jr (2001) The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res 61:3369–3372
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Eng J Med 355:2542–2550
Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, Horsfield MA, Mross K, Ball HA, Lee L, Mietlowski W, Fuxuis S, Unger C, O’Byrne K, Henry A, Cherryman GR, Laurent D, Dugan M, Marme D, Steward WP (2003) DCE-MRI as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 21:3955–3964
Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, Masson E, Puccio-Pick M, Laurent D, Steward WP (2005) Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol 23:4162–4171
PTK787/ZK 222584 (2006) Investigator’s Brochure edn. No. 8
Flaherty KT, Schiller J, Schuchter LM, Liu G, Tuveson DA, Redlinger M, Lathia C, Xia C, Petrenciuc O, Hingorani SR, Jacobetz MA, Van Belle PA, Elder D, Brose MS, Weber BL, Albertini MR, O’Dwyer PJ (2008) A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res 14:4836–4842
Okamoto I, Miyazaki M, Morinaga R, Kaneda H, Ueda S, Hasegawa Y, Satoh T, Kawada A, Fukuoka M, Fukino K, Tanigawa T, Nakagawa K (2009) Phase I clinical and pharmacokinetic study of sorafenib in combination with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Invest New Drugs. doi: 10.1007/s10637-009-9321-x
Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D, Robertson J, Puchalski TA, Seymour L (2008) Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer: the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 26:1871–1878
Thomas AL, Trarbach T, Bartel C, Laurent D, Henry A, Poethiq M, Wang J, Masson E, Steward W, Vanhoefer U, Wiedenmann B (2007) A phase IB, open-label dose escalating study of the oral angiogenesis inhibitor PTK787/ZK 222584 (PTK/ZK), in combination with FOLFOX4 chemotherapy in patients with advanced colorectal cancer. Ann Oncol 18:782–788
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Venook AP, Egorin MJ, Rosner GL, Brown TD, Jahan TM, Batist G, Hohl R, Budman D, Ratain MJ, Kearns CM, Schilsky RL (1998) Phase I and pharmacokinetic trial of paclitaxel in patients with hepatic dysfunction: Cancer and Leukemia Group B 9264. J Clin Oncol 16:1811–1819
Joerger M, Huitema AD, Huizing MT, Willemse PH, de Graeff A, Rosing H, Schellens JH, Beijnen JH, Vermorken JB (2007) Safety and pharmacology of paclitaxel in patients with impaired liver function: a population pharmacokinetic-pharmacodynamic study. Br J Clin Pharmacol 64:622–633
Jost LM, Gschwind HP, Jalava T, Wang Y, Guenther C, Souppart C, Rottmann A, Denner K, Waldmeier F, Gross G, Masson E, Laurent D (2006) Metabolism and disposition of Vatalanib (PTK787/ZK-222584) in cancer patients. Drug Metab Disp 34:1817–1828
Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D, Dunn SA, Gilissen R, Houdt JV, Hendrickx J, Mannens G, Bohets H, Williams FM, Armstrong M, Crespi CL, Daly AK (2002) CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem Pharmacol 64:1579–1589
Taniguchi R, Kumai T, Matsumoto N, Watanabe M, Kamio K, Suzuki S, Kobayashi S (2005) Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci 97:83–90
Marsh S, Somlo G, Li X, Frankel P, King CR, Shannon WD, McLeod HL, Synold TW (2007) Pharmacogenetic analysis of paclitaxel transport and metabolism genes in breast cancer. Pharmacogenomics 7:362–365
Fennelly D, Aghajanian C, Shapiro F, O’Flaherty C, McKenzie M, O’Connor C, Tong W, Norton L, Spriggs D (1997) Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer. J Clin Oncol 15:187–192
Sonnichsen DS, Relling MV (1994) Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet 27:256–269
Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, Vermorken JB, Strocchi E, Martoni A, Sprio R, Sleeboom HP, Izquierdo MA, Jodrell DI, Calvert H, Boddy AV, Hollema H, Fety R, Van der Vijgh WJ, Hempel G, Chatelut E, Karlsson M, Wilkins J, Tranchand B, Schrijvers AH, Twelves C, Beijnen JH, Schellens JH (2007) Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European Organization for Research and Treatment of Cancer-Pharmacology and Molecular Mechanisms Group and New Drug Development Group. Clin Cancer Res 13:6410–6418
Huizing MT, Giaccone G, van Warmerdam LJ, Rosing H, Bakker PJ, Vermorken JB, Postmus PE, van Zandwijk N, Koolen MG, ten Bokkel Huinink WW, van der Vijgh WJ, Bierhorst FJ, Lai A, Dalesio O, Pinedo HM, Veenhof CH, Beijnen JH (1997) Pharmacokinetics pf paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small cell lung cancer. J Clin Oncol 15:317–329
Gonzales-Angulo AM, Hortobagyi GN (2008) Optimal schedule of paclitaxel: weekly is better. J Clin Oncol 26:1585–1586
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Novartis Pharmaceuticals; General Clinical Research Center at Indiana University School of Medicine M01RR00750.
Rights and permissions
About this article
Cite this article
Chiorean, E.G., Malireddy, S., Younger, A.E. et al. A phase I dose escalation and pharmacokinetic study of vatalanib (PTK787/ZK 222584) in combination with paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 66, 441–448 (2010). https://doi.org/10.1007/s00280-009-1179-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1179-2