Abstract
Purpose
Pegylated liposomal doxorubicin (PLD, CAELYX®) has demonstrated activity in several phase-III trials and has been approved for the therapy of relapsed ovarian cancer after platinum treatment. Aim of this observational study was to analyze the efficacy and toxicity profile of PLD under routine clinical conditions and without the general restrictions of defined inclusion and exclusion criteria of clinical trials.
Methods
Between 2003 and 2005, a total of 190 patients with relapsed ovarian cancer were enrolled. 183 patients were available for evaluation; dose-intensity, modifications, treatment duration, toxicities and response were systematically analyzed.
Results
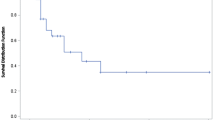
The median patient age was 62 years (range 23–86 years). 45.4% of the patients received PLD as second-line therapy and a median of four courses per patient were administered. The median dose of PLD was 40 mg/m2, most frequently used every 4 weeks (68.8%). Grade 3 Leucopenia (1.6%) and grade 3 and 4 thrombocytopenia (0.5%) were the most frequent hematological toxicities. The most frequent non-hematological toxicities were skin toxicity, pain and nausea, which were observed in 38.8, 41 and 45.9% of the patients, respectively. Twenty-seven percent of the patients showed a response to therapy with 6.9% achieving complete remission and 20.1% achieving partial remission. 37.7% achieved a stable disease. The median duration of response for all patients was 4.8 months (range 0–51.8 months). Median progression-free interval and overall survival were 5.8 months (95% CI 5.1–6.6 months) and 16.6 months (95% CI 13.9–22.6 months), respectively.
Conclusions
PLD is safe and effective in patients with relapsed ovarian cancer, even after numerous previous treatment regimens. A dose of 40 mg/m2 every 28 days seems to be an effective and well-tolerated therapeutic option in advanced ovarian cancer with a low incidence of hematological toxicities and acceptable non-hematological toxicities.

Similar content being viewed by others
Abbreviations
- BfArM:
-
German Federal Institute for Drugs and Medical Devices
- CI:
-
Confidence interval
- CR:
-
Complete remission
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- ECOG:
-
Eastern Cooperative Oncology Group
- nd:
-
Not determined
- NOGGO:
-
North-Eastern German Society of Gynecological Oncology
- PD:
-
Progressive disease
- PLD:
-
Pegylated liposomal doxorubicin
- PPE:
-
Palmar-plantar erythrodysesthesia
- PR:
-
Partial remission
- SAE:
-
Serious adverse events
- SD:
-
Stable disease
References
Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Tropé C (2003) ICON ans AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR 2.2 trial. Lancet 361(9375):2099–2106
McGuire WP (2001) Primary therapy of epithelial ovarian cancer. In: Perry MC (Ed) American society of clinical oncology educational book, pp 477–480
Al-Batran SE, Bischoff J, von Minckwitz G, Atmaca A, Kleeberg U, Meuthen I, Morack G, Lerbs W, Hecker D, Sehouli J, Knuth A, Jager E (2006) The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: a multicentre phase II trial. Br J Cancer 94:1615–1620
Vermorken JB (2003) The role of anthracyclines in second-line therapy of ovarian cancer. Int J Gynecol Cancer 13:178–184
Gabizon AA (1994) Liposomal anthracyclines. Hematol Oncol Clin North Am 8:431–450
Martin FJ, Gabizon A (1992) Human pharmacokinetics of stealth liposomes containing doxorubicin. J Cell Biochem 16E(Suppl):98
Gordon AN, Fleagle JT, Guthrie D, Parkin DR, Gore ME, Lacave AJ (2001) Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19:3312–3322
Gordon AN, Tonda M, Sun S, Rackoff W, Doxil Study 30–49 Investigators (2004) Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol 95:1–8
Valerio MR, Tagliaferrri P, Raspagliesi F, Fulfaro F, Badalamenti G, Arcara C, Cicero G, Russo A, Venuta S, Guarneri G, Gebbia N (2006) A phase II study of pegylated liposomal doxorubicin oxaliplatin and cyclophosphamide as second-line treatment in relapsed ovarian carcinoma. Int J Gynecol Cancer 16(Suppl 1):79–85
Ten Bokkel Huinink W, Lane SR, Ross GA (2004) Long term survival in a phase III, randomized study of topotecan versus paclitaxel in advanced epithelial ovarian carcinoma. Ann Oncol 15:100–103
Rose PG (2000) Phase I study of paclitaxel, carboplatin, and liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a retrospective comparative study of single-agent dosages. Gynecol Oncol 82:323–328
Rose PG, Maxson JH, Fusco N, Mossbruger K, Rodriguez M (2001) Liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a retrospective comparative study of single-agent dosages. Gynecol Oncol 82:323–328
Hackbarth M, Haas H, Fotopoulou C, Sehouli J (2007) Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in womens’ cancers: results of a prospective study. Support Care Cancer 16(3):267–273
von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, Sehouli J, Scotte F, Lorusso D, Dummer R, Lacouture ME, Lademann J, Hauschild A (2008) Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer 44(6):781–790
Sehouli J, Oskay-Ozcelik G, Kuhne J, Stengel D, Hindenburg HJ, Klare P, Heinrich G, Schmalfeldt B, Mertens H, Camara O, Lichtenegger W (2006) Biweekly pegylated liposomal doxorubicin in patients with relapsed ovarian cancer: results of a multicenter phase-II trial. Ann Oncol 17:957–961
Oskay-Oezcelik G, Koensgen D, Hindenburg H-J, Klare P, Schmalfeldt B, Lichtenegger W, Chekerov R, Al-Batran SE, Neumann U, Sehouli J (2008) Biweekly pegylated liposomal doxorubicin as second line treatment in patients with relapsed ovarian cancer after failure of platinum and paclitaxel: results from a multi-center phase II study of the NOGGO. Anticancer Res 28(2B):1329–1334
Acknowledgments
The authors gratefully acknowledge all of the many patients and their families, as well as clinical investigators and their study center trial facility staff who supported this trial. Without their enthusiastic collaboration this work would not have been possible. Furthermore, we would like to thank the following institutions for their contributions to this trial: Medical Practice Dr. Prechtl (München), Medical Practice Dr. Neise (Krefeld), Medical Practice Dr. Nusch (Velbert), Medical Practice Dr. Kurbacher (Bonn), Medical Practice Dr. Göhler (Dresden), Medical Practice Dr. Lerchenmüller (Münster), Medical Practice Dr. Reschke (Oldenburg), Department of Gynecology and Obstetrics—Hospital Augsburg Prof. Wischnik, Medical Practice Dr. Fett (Wuppertal), Medical Practice Dr. Selbach (Duisburg), Medical Practice Dr. Bittrich (Erfurt), Medical Practice Dr. Hempel (Rehling), Department of Gynecology and Obstetrics—Hospital Heidelberg Prof. Bastert, Medical Practice Dr. Busch (Mühlhausen), Medical Practice Dr. Steffen (Gera), Medical Practice Dr. Rohrberg (Halle), Medical Practice Dr. Kasper (Hof), Department of Gynecology and Obstetrics—Hospital Krefeld Prof. Baltzer, Medical Practice Dr. Hälbig (Eisenach), Medical Practice Dr. Wiegand (Moers), Medical Practice Dr. Mainz (Würselen), Medical Practice Dr. Schardt Gelsenkirchen, Medical Practice Dr. Tessen Goslar, Medical Practice Dr. Heinrich (Fürstenwalde), Medical Practice Dr. Mittermüller (Germering), Medical Practice Dr. Schick (München), Medical Practice Dr.Wierick (Weißkollm), Medical Practice Dr. Blumenstengel (Eisenach), Medical Practice Dr. Perker (Weilheim), Medical Practice Dr. Stauch (Kronach), Medical Practice Dr. Schneider (Neusäß), Medical Practice Dr. Tummes (Aachen), Medical Practice Dr. Weinberg (Aachen), Medical Practice Dr. Schlag (Würzburg), Medical Practice Dr. Schwindt (Bonn).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Sehouli, J., Camara, O., Schmidt, M. et al. Pegylated liposomal doxorubicin (CAELYX®) in patients with advanced ovarian cancer: results of a German multicenter observational study. Cancer Chemother Pharmacol 64, 585–591 (2009). https://doi.org/10.1007/s00280-008-0909-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0909-1