Abstract
Purpose
Suberonylanilide hydroxamic acid (SAHA) is an orally administered histone deacetylase inhibitor (HDACI) that has shown significant antitumor activity in a variety of tumor cells. To evaluate if SAHA has an activity against liver cancer, and with an aim to identify the altered cellular factors upon SAHA treatment, human HepG2 cancer cell line was used as a model, and proteomic approach was utilized to elucidate the molecular mechanisms underlying SAHA’s antitumor activity.
Methods
Cell growth inhibition was measured by MTT method, and apoptosis was detected by means of flow cytometry analysis and TUNEL assay. Protein expression profiles were analyzed by 2-DE coupled with MALDI-Q-TOF MS/MS analysis.
Results
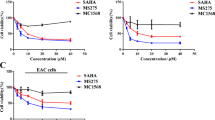
A total of 55 differentially expressed proteins were visualized by 2-DE and Coomassie Brilliant Blue (CBB) staining. Of these, 34 proteins were identified via MS/MS analysis. Among the identified proteins, six proteins also displayed significant expression changes at earlier time points upon SAHA treatment, and such alterations were further confirmed by semi-quantitative RT-PCR. Together, at both the mRNA and protein levels, SAHA suppressed the expression of reticulocalbin 1 precursor (RCN1), annexin A3 (ANXA3) and heat shock 27 kDa protein 1 (HSP27), while increasing the expression of aldose reductase (AR), triosephosphate isomerase 1 (TPI) and manganese superoxide dismutase (SOD2).
Conclusion
SAHA remarkably inhibited proliferation of HepG2 cancer cells, and induced apoptosis in vitro. Using proteomics approaches, a variety of differentially expressed proteins were identified in HepG2 cancer cells before and after treatment with SAHA. This study will enable a better understanding of the molecular mechanisms underlying SAHA-mediated antitumor effects at the protein level.






Similar content being viewed by others
Abbreviations
- SAHA:
-
Suberonylanilide hydroxamic acid
- HADC:
-
Histone deacetylase
- HADCIs:
-
Histone deacetylase inhibitors
- 2-DE:
-
2-Dimensional polyacrylamide gel electrophoresis
- HCC:
-
Hepatocellular carcinoma
- RCN1:
-
Reticulocalbin 1
- ANXA3:
-
Annexin A3
- HSP27:
-
Heat shock 27 kDa protein 1
- AR:
-
Aldose reductase
- TPI:
-
Triosephosphate isomerase 1
- SOD2:
-
Manganese superoxide dismutase
References
Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA et al (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA 95:3003–3007
Narlikar GJ, Fan HY, Kingston RE (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475–487
Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C et al (2000) Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 60:5165–5170
Cao ZA, Bass KE, Balasubramanian S, Liu L, Schultz B, Verner E et al (2006) CRA-026440: a potent, broad-spectrum, hydroxamic histone deacetylase inhibitor with antiproliferative and antiangiogenic activity in vitro and in vivo. Mol Cancer Ther 5:1693–1701
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA et al (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401:188–193
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1:194–202
Richon VM, Sandhoff TW, Rifkind RA, Marks PA (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA 97:10014–10019
Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R et al (2001) The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA 98:10833–10838
Henderson C, Mizzau M, Paroni G, Maestro R, Schneider C, Brancolini C et al (2003) Role of caspases, Bid, and p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin: a (TSA) and suberoylanilide hydroxamic acid (SAHA). J Biol Chem 278:12579–12589
Takada Y, Gillenwater A, Ichikawa H, Aggarwal BB (2006) Suberoylanilide hydroxamic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing nuclear factor-kappaB activation. J Biol Chem 281:5612–5622
Xu W, Ngo L, Perez G, Dokmanovic M, Marks PA (2006) Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitor. Proc Natl Acad Sci USA 103:15540–15545
El-Serag HB, Davila JA, Petersen NJ, McGlynn KA (2003) The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 139:817–823
Wilson JF (2005) Liver cancer on the rise. Ann Intern Med 142:1029–1032
Cormier JN, Thomas KT, Chari RS, Pinson CW (2006) Management of hepatocellular carcinoma. J Gastrointest Surg 10:761–780
Herold C, Ganslmayer M, Ocker M, Hermann M, Geerts A, Hahn EG et al (2002) The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J Hepatol 36:233–240
Yamashita Y, Shimada M, Harimoto N, Rikimaru T, Shirabe K, Tanaka S et al (2003) Histone deacetylase inhibitor trichostatin A induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. Int J Cancer 103:572–576
Armeanu S, Pathil A, Venturelli S, Mascagni P, Weiss Thomas S, Gottlicher M et al (2005) Apoptosis on hepatoma cells, but not on primary hepatocytes by histone deacetylase inhibitors valproate and ITF2357. J Hepatol 42:210–217
Jain KK (2000) Applications of proteomics in oncology. Pharmacogenomics 1:385–393
Kabuyama Y, Resing KA, Ahn NG (2004) Applying proteomics to signaling networks. Curr Opin Genet Dev 14:492–498
Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM et al (2006) Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int 70:496–506
Sakajiri S, Kumagai T, Kawamata N, Saitoh T, Said JW, Koeffler HP (2005) Histone deacetylase inhibitors profoundly decrease proliferation of human lymphoid cancer cell lines. Exp Hematol 33:53–61
Song H, Ethier SP, Dziubinski ML, Lin J (2004) Stat3 modulates heat shock 27 kDa protein expression in breast epithelial cells. Biochem Biophys Res Commun 314:143–150
Neo JCH, Rose P, Ong CN, Chung MCM (2005) Beta-phenylethyl isothiocyanate mediated apoptosis: a proteomic investigation of early apoptotic protein changes. Proteomics 5:1075–1082
Gray SG, Qian CN, Furge K, Guo X, Teh BT (2004) Microarray profiling of the effects of histone deacetylase inhibitors on gene expression in cancer cell lines. Int J Oncol 24:773–795
Ozawa M, Muramatsu T (1993) Reticulocalbin, a novel endoplasmic reticulum resident Ca(2+)-binding protein with multiple EF-hand motifs and a carboxyl-terminal HDEL sequence. J Biol Chem 268:699–705
Tachikui H, Navet AF, Ozawa M (1997) Identification of the Ca(2+)-binding domains in reticulocalbin, an endoplasmic reticulum resident Ca(2+)-binding protein with multiple EF-hand motifs. J Biochem (Tokyo) 121:145–149
Kent J, Lee M, Schedl A, Boyle S, Fantes J, Powell M et al (1997) The reticulocalbin gene maps to the WAGR region in human and to the small eye Harwell deletion in mouse. Genomics 42:260–267
Yu LR, Zeng R, Shao XX, Wang N, Xu YH, Xia QC (2000) Identification of differentially expressed proteins between human hepatoma and normal liver cell lines by two-dimensional electrophoresis and liquid chromatography-ion trap mass spectrometry. Electrophoresis 21:3058–3068
Liu Z, Brattain MG, Appert H (1997) Differential display of reticulocalbin in the highly invasive cell line, MDA-MB-435, versus the poorly invasive cell line, MCF-7. Biochem Biophys Res Commun 231:283–289
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371
Hsiang CH, Tunoda T, Whang YE, Tyson DR, Ornstein DK (2006) The impact of altered annexin I protein levels on apoptosis and signal transduction pathways in prostate cancer cells. Prostate 66:1413–1424
Niimi S, Harashima M, Gamou M, Hyuga M, Seki T, Ariga T et al (2005) Expression of annexin A3 in primary cultured parenchymal rat hepatocytes and inhibition of DNA synthesis by suppression of annexin A3 expression using RNA interference. Biol Pharm Bull 28:424–428
Ciocca DR, Calderwood SK (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10:86–103
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172
Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E (1999) HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J 13:2061–2070
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T et al (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278:27828–27835
Kammanadiminti SJ, Chadee K (2006) Suppression of NF-kappaB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J Biol Chem 281:26112–26120
Pacey S, Banerji U, Judson I, Workman P (2006) Hsp90 inhibitors in the clinic. Handb Exp Pharmacol 172:331–358
Guo Z, Boekhoudt GH, Boss JM (2003) Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganous superoxide dismutase. J Biol Chem 278:23570–23578
Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS et al (1993) Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci USA 90:3113–3117
Weydert C, Roling B, Liu J, Hinkhouse MM, Ritchie JM, Oberley LW et al (2003) Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther 2:361–369
Venkataraman S, Jiang X, Weydert C, Zhang Y, Zhang HJ, Goswami PC et al (2005) Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene 24:77–89
Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L et al (2006) Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic Biol Med 41:226–237
Hodge DR, Xiao W, Peng B, Cherry JC, Munroe DJ, Farrar WL (2005) Enforced expression of superoxide dismutase 2/manganese superoxide dismutase disrupts autocrine interleukin-6 stimulation in human multiple myeloma cells and enhances dexamethasone-induced apoptosis. Cancer Res 65:6255–6263
Yabe-Nishimura C (1998) Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev 50:21–33
Hamaoka R, Fujii J, Miyagawa J, Takahashi M, Kishimoto M, Moriwaki M et al (1999) Overexpression of the aldose reductase gene induces apoptosis in pancreatic beta-cells by causing a redox imbalance. J Biochem (Tokyo) 126:41–47
Galvez AS, Ulloa JA, Chiong M, Criollo A, Eisner V, Barros LF et al (2003) Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol. J Biol Chem 278:38484–38494
Ramana KV, Bhatnagar A, Srivastava SK (2004) Inhibition of aldose reductase attenuates TNF-alpha-induced expression of adhesion molecules in endothelial cells. FASEB J 18:1209–1218
Murata M, Ohta N, Sakurai S, Alam S, Tsai J, Kador PF et al (2001) The role of aldose reductase in sugar cataract formation: aldose reductase plays a key role in lens epithelial cell death (apoptosis). Chem Biol Interact 130–132:617–625
Miwa K, Nakamura J, Hamada Y, Naruse K, Nakashima E, Kato K et al (2003) The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res Clin Pract 60:1–9
Gess B, Hofbauer KH, Deutzmann R, Kurtz A (2004) Hypoxia up-regulates triosephosphate isomerase expression via an HIF-dependent pathway. Pflugers Arch 448:175–180
Jiang PZ, Gan M, Huang H, Shen XM, Wang S, Yao KT (2005) Proteomics-based identification of proteins with altered expression induced by 12-O-tetradecanoylphorbol 13-acetate in nasopharyngeal carcinoma CNE2 cells. Acta Biochim Biophys Sin (Shanghai) 37:97–106
Lieu HY, Song HS, Yang SN, Kim JH, Kim HJ, Park YD et al (2006) Identification of proteins affected by iron in Saccharomyces cerevisiae using proteome analysis. J Microbiol Biotechnol 16:946–951
Richon VM (2006) Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor.95(Suppl 1):S2–S6
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784
Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6:38–51
Lescuyer P, Hochstrasser DF, Sanchez JC (2004) Comprehensive proteome analysis by chromatographic protein prefractionation. Electrophoresis 25:1125–1135
Corthals GL, Wasinger VC, Hochstrasser DF, Sanchez JC (2000) The dynamic range of protein expression: a challenge for proteomic research. Electrophoresis 21:1104–1115
Zhang CL, Richon V, Ni X, Talpur R, Duvic M (2005) Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol 125:1045–1052
Huang C, Ida H, Ito K, Zhang H, Ito Y (2007) Contribution of reactivated RUNX3 to inhibition of gastric cancer cell growth following suberoylanilide hydroxamic acid (vorinostat) treatment. Biochem Pharmacol 73:990–1000
Sonnemann J, Gange J, Pilz S, Stotzer C, Ohlinger R, Belau A et al (2006) Comparative evaluation of the treatment efficacy of suberoylanilide hydroxamic acid (SAHA) and paclitaxel in ovarian cancer cell lines and primary ovarian cancer cells from patients. BMC Cancer 6:183
Acknowledgments
This work was supported by grants from the National 973 Basic Research Program of China (2004CB518807, 2006CB504303 and 2006CB504302) and the Sichuan Applied Basic Research (07JY029-052).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tong, A., Zhang, H., Li, Z. et al. Proteomic analysis of liver cancer cells treated with suberonylanilide hydroxamic acid. Cancer Chemother Pharmacol 61, 791–802 (2008). https://doi.org/10.1007/s00280-007-0536-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0536-2