Abstract
Objectives
The aim of this phase II study was to evaluate the response rate to gemcitabine combined with cisplatin in patients with locally advanced, metastatic or recurrent biliary tract cancer who had received no prior chemotherapy.
Methods
The treatment consisted of cisplatin 70 mg/m2 in intravenous infusion followed by gemcitabine 1,250 mg/m2 in 30-min intravenous infusion on days 1 and 8, repeated every 3 weeks until disease progression, unacceptable toxicity, patient’s refusal or up to 8 cycles.
Results
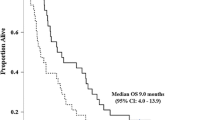
Thirty-nine patients with advanced biliary cancer were enrolled between March 2003 and August 2003. Fourteen patients (40%) had gall bladder cancer and 20 patients (57%) had cholangiocarcinoma. Thirty-two patients (91%) had metastatic disease at study entry with liver being the most commonly involved site of metastasis. About 84.5 and 94.2% of the initially planned dose were administered for gemcitabine and cisplatin, respectively. In the ITT population (n = 35), six partial responses were observed for an objective response rate of 17.1% (95% CI; 4.7–29.6%). Ten patients (28.6%) had stable disease, 16 (45.7%) progressed, and three (8.6%) were not evaluable. For the 35 patients in the ITT population, the median overall survival time was 8.6 months (95% CI; 6.1–10.4 months). The median time to disease progression was 3.2 months (95% CI; 2.3–4.9 months) and the median time to treatment failure was 3.1 months (95% CI; 1.9–4.1 months). Among the six tumor responders, the median duration of tumor response was 7.3 months (95% CI; 5.6–11.0 months). The most common grade 3/4 maximum toxicities were nausea (3.4%) and vomiting (2.7%).
Conclusion
The combination chemotherapy with gemcitabine and cisplatin in this trial demonstrated moderate antitumor activity with favorable toxicity profile.

Similar content being viewed by others
References
Shin HR, Jung JK, Won YJ, Park JG (2004) 139 KCCR-affiliated Hospitals. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat 36:103–114
Patel T (2002) Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2:10
Oertli D, Herzog U, Tondelli P (1993) Primary carcinoma of the gallbladder: operative experience during a 16 year period. Eur J Surg 159(8):415–420
Alexander F, Rossi RL, O’Bryan M, Khettry U, Braasch JW, Watkins E Jr (1984) Biliary carcinoma. A review of 109 cases. Am J Surg 147(4):503–509
Wade TP, Prasad CN, Virgo KS, Johnson FE (1997) Experience with distal bile duct cancers in US. Veterans Affairs hospitals: 1987–1991. J Surg Oncol 64(3):242–245
de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM (1999) Biliary tract cancers. N Engl J Med 341(18):1368–1378
Scheithauer W (2002) Review of gemcitabine in biliary tract carcinoma. Semin Oncol 29(6 Suppl 20):40–45
Arroyo G, Gallardo J, Rubio B, et al (2001) Gemcitabine (GEM) in advanced biliary tract cancer (ABTC). Experimence from Chile and Argentina in phase II trials. Proc Am Soc Clin Oncol 20:A626
Gebbia V, Giuliani F, Maiello E, Colucci G, Verderame F, Borsellino N, et al (2001) Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol 19(20):4089–4091
Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, et al (2001) Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 12(2):183–186
Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ (1996) Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 2(3):521–530
Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ (1995) Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol 22(4 Suppl 11):72–79
Carraro S, Servienio P, Bruno MF, Castillo Odena MDS, Roca E, Jovtis S, Felci N, Araujo C (2001) Gemcitabine and cisplatin in locally advanced or metastatic gallbladder and bile duct adenocarcinomas. Proc Am Soc Clin Oncol 20:A2333
Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, et al (2004) A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer 90(8):1516–1520
Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS (2006) Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol 29(2):127–131
Thongprasert S, Napapan S, Charoentum C, Moonprakan S (2005) Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol 16(2):279–281
Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al (2006) A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 106(6):1339–1346
Reyes-Vidal J GJ, Cerda B et al (2003) Gemcitabine and cisplatin in the treatment of patients with unresectable or metastatic gallbladder cancer: results of the phase II GOCCHI study 2000–13. Proc Am Soc Clin Oncol 22:273:1095 (Abstract)
Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA (2005) Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 93(2):195–199
Cho JY, Nam JS, Park MS, Yu JS, Paik YH, Lee SJ, et al (2005) A Phase II study of capecitabine combined with gemcitabine in patients with advanced gallbladder carcinoma. Yonsei Med J 46(4):526–531
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, et al (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23(10):2332–2338
Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 15(9):1339–1343
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Supported by Korean Cancer Study Group.
Rights and permissions
About this article
Cite this article
Lee, J., Kim, TY., Lee, M.A. et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol 61, 47–52 (2008). https://doi.org/10.1007/s00280-007-0444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0444-5