Abstract
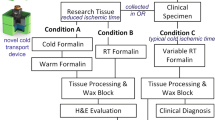
Demonstration of pharmacodynamic activity of new, targeted cancer drugs in tumour tissue is potentially important in guiding early drug development. However, delays between tumour sampling and sample fixation may result in variability of pharmacodynamic biomarkers. The aim of this study, was to assess the impact of delays in fixation on biomarkers of Src kinase activity. A total of 20 patients with locally advanced breast cancer and 5 with early bladder cancer had multiple tissue samples taken which were fixed at documented time points up to 60 min after biopsy. These were examined to determine if the amount of Paxillin, phospho-Paxillin, phospho-focal adhesion kinase (FAK) and total phospho-Tyrosine changed over time, using a quantitative lysate immunoassay. In breast cancer, there was an increase in the amount of phospho-Paxillin (60% per h; P = 0.019) up to 60 min after biopsy. The amount of total Paxillin decreased (28% per h; P = 0.034) over the same time course. In early bladder cancer, no changes were noted in any endpoints up to 45 min. Standardisation of the time taken between biopsy and fixation may be critical, particularly in studies using phosphorylated protein biomarkers.



Similar content being viewed by others
References
Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, Schiffer CA, Fischer T, Deininger MW, Lennard AL, Hochhaus A, Ottmann OG, Gratwohl A, Baccarani M, Stone R, Tura S, Mahon FX, Fernandes-Reese S, Gathmann I, Capdeville R, Kantarjian HM, Sawyers CL (2002) Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99:1928–1937
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364:1127–1134
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L (2005) National Cancer Institute of Canada Clinical Trials Group: Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Green T, Hennequin LR, Ple PA, Jones RJ, Clack G, Gallagher N: Pre-clinical and early clinical activity of the highly selective, orally available, dual Src/Abl kinase inhibitor AZD0530 (abstract). ‘New drugs on the horizon’, 2005 AACR Annual Meeting, Anaheim
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM (2004) Discovery of N-(2-Chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin–4-ylamino)thiazole-5-carboxamide (BMS-354825), a Dual Src/Abl Kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47:6658–6661
Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602:114–130
Ple PA, Green TP, Hennequin LF, Curwen J, Fennell M, Allen J, Lambert-Van Der Brempt C, Costello G (2004) Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src. J Med Chem 47:871–887
Kawakatsu H, Sakai T, Takagaki Y, Shinoda Y, Saito M, Owada MK, Yano J (1996) A new monoclonal antibody which selectively recognizes the active form of Src tyrosine kinase. J Biol Chem 271:5680–5685
Paronetto MP, Farini D, Sammarco I, Maturo G, Vespasiani G, Geremia R, Rossi P, Sette C (2004) Expression of a truncated form of the c-Kit tyrosine kinase receptor and activation of Src kinase in human prostatic cancer. Am J Pathol 164:1243–1251
Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE (2002) Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer 94:344–351
Esteva FJ, Sahin AA, Smith TL, Yang Y, Pusztai L, Nahta R, Buchholz TA, Buzdar AU, Hortobagyi GN, Bacus SS (2004) Prognostic significance of phosphorylated P38 mitogen-activated protein kinase and HER-2 expression in lymph node-positive breast carcinoma. Cancer 100:499–506
Massion PP, Taflan PM, Shyr Y, Rahman SM, Yildiz P, Shakthour B, Edgerton ME, Ninan M, Andersen JJ, Gonzalez AL (2004) Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med 170:1088–1094
Lim J, Kim JH, Paeng JY, Kim MJ, Hong SD, Lee JI, Hong SP (2005) Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol 58:1199–1205
Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S (2005) Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer 103:307–312
Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Palmer AA, Zhang XD, Thompson JF, Bron LP, Hersey P (2005) Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma. J Clin Pathol 58:1163–1169
Handra-Luca A, Bilal H, Bertrand JC, Fouret P (2003) Extra-cellular signal-regulated ERK-1/ERK-2 pathway activation in human salivary gland mucoepidermoid carcinoma: association to aggressive tumor behavior and tumor cell proliferation. Am J Pathol 163:957–967
Etges A, Pinto DS, Kowalski LP, Soares FA, Araujo VJ (2003) Salivary duct carcinoma: immunohistochemical profile of an aggressive salivary gland tumour. Clin Pathol 56:914–918
Sivridis E, Giatromanolaki A (2004) Proliferative activity in postmenopausal endometrium: the lurking potential for giving rise to an endometrial adenocarcinoma. J Clin Pathol 57:840–844
Prasad R, Boland GP, Cramer A, Anderson E, Knox WF, Bundred NJ (2003) Short-term biologic response to withdrawal of hormone replacement therapy in patients with invasive breast carcinoma. Cancer 98:2539–2546
Rodrigues NA, Dillon D, Carter D, Parisot N, Haffty BG (2003) Differences in the pathologic and molecular features of intraductal breast carcinoma between younger and older women. Cancer 97:1393–1403
Michels JJ, Duigou F, Marnay J, Henry-Amar M, Delozier T, Denoux Y, Chasle J (2003) Flow cytometry and quantitative immunohistochemical study of cell cycle regulation proteins in invasive breast carcinoma: prognostic significance. Cancer 97:1376–1386
Pich A, Chiusa L, Formiconi A, Galliano D, Bortolin P, Comino A, Navone R (2002) Proliferative activity is the most significant predictor of recurrence in non-invasive papillary urothelial neoplasms of low malignant potential and grade 1 papillary carcinomas of the bladder. Cancer 95:784–790
Wohlschlegel JA, Kutok JL, Weng AP, Dutta A (2002) Expression of geminin as a marker of cell proliferation in normal tissues and malignancies. Am J Pathol 161:267–273
Kouvaraki M, Gorgoulis VG, Rassidakis GZ, Liodis P, Markopoulos C, Gogas J, Kittas C (2002) High expression levels of p27 correlate with lymph node status in a subset of advanced invasive breast carcinomas: relation to E-cadherin alterations, proliferative activity, and ploidy of the tumors. Cancer 94:2454–2465
Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, Niedobitek G, Brabletz T, Kirchner T (2001) The invasion front of human colorectal adenocarcinomas shows co-localisation of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol 159:1613–1617
Kawauchi S, Goto Y, Liu XP, Furuya T, Oga A, Oda Y, Tsuneyoshi M, Ihara K, Sasaki K (2001) Low expression of p27(Kip1), a cyclin-dependent kinase inhibitor, is a marker of poor prognosis in synovial sarcoma. Cancer 91:1005–1012
Vis AN, Noordzij MA, Fitoz K, Wildhagen MF, Schroder FH, van der Kwast TH (2000) Prognostic value of cell cycle proteins p27(kip1) and MIB-1, and the cell adhesion protein CD44s in surgically treated patients with prostate cancer. J Urol 164:2156–2161
Noguchi T, Dobashi Y, Minehara H, Itoman M, Kameya T (2000) Involvement of cyclins in cell proliferation and their clinical implications in soft tissue smooth muscle tumors. Am J Pathol 156:2135–2147
Antonescu CR, Leung DH, Dudas M, Ladanyi M, Brennan M, Woodruff JM, Cordon-Cardo C (2000) Alterations of cell cycle regulators in localised synovial sarcoma: a multifactorial study with prognostic implications. Am J Pathol 156:977–983
de Alava E, Panizo A, Antonescu CR, Huvos AG, Pardo-Mindan FJ, Barr FG, Ladanyi M (2000) Association of EWS-FLI1 type 1 fusion with lower proliferative rate in Ewing’s sarcoma. Am J Pathol 156:849–855
Migita T, Oda Y, Naito S, Tsuneyoshi M (2002) Low expression of p27(Kip1) is associated with tumor size and poor prognosis in patients with renal cell carcinoma. Cancer 94:973–979
Reis-Filho JS, Faoro LN, Carrilho C, Bleggi-Torres LF, Schmitt FC (2000) Evaluation of cell proliferation, epidermal growth factor receptor, and bcl-2 immunoexpression as prognostic factors for patients with World Health Organization grade 2 oligodendroglioma. Cancer 88:862–869
Sakurai K, Urade M, Takahashi Y, Kishimoto H, Noguchi K, Yasoshima H, Kubota A (2000) Increased expression of c-erbB-3 protein and proliferating cell nuclear antigen during development of verrucous carcinoma of the oral mucosa. Cancer 89:2597–2605
Haitel A, Posch B, El-Baz M, Mokhtar AA, Susani M, Ghoneim MA, Marberger M (2001) Bilharzial related, organ confined, muscle invasive bladder cancer: prognostic value of apoptosis markers, proliferation markers, p53, E-cadherin, epidermal growth factor receptor and c-erbB-2. J Urol 165:1481–1487
Lake-Bakaar G, Mazzoccoli V, Ruffini L (2002) Digital image analysis of the distribution of proliferating cell nuclear antigen in hepatitis C virus-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci 47:1644–1648
Noda H, Maehara Y, Irie K, Kakeji Y, Yonemura T, Sugimachi K (2002) Increased proliferative activity caused by loss of p21(WAF1/CIP1) expression and its clinical significance in patients with early-stage gastric carcinoma. Cancer 94:2107–2112
Spyratos F, Ferrero-Pous M, Trassard M, Hacene K, Phillips E, Tubiana-Hulin M, Le Doussal V (2002) Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer 94:2151–2159
Mizutani Y, Wada H, Yoshida O, Fukushima M, Kamoi K, Miki T (2002) Prognostic significance of thymidine kinase activity in bladder carcinoma. Cancer 95:2120–2125
Boltze C, Mundschen J, Unger N, Schneider-Stock R, Peters B, Mawrin C, Hoang-Vu C, Roessner A, Lehnert H (2003) Expression profile of the telomeric complex discriminates between benign and malignant pheochromocytoma. J Clin Endocrinol Metab 88:4280–4286
Bravaccini S, Sanchini MA, Amadori A, Medri L, Saragoni L, Calistri D, Monti F, Volpi A, Amadori D (2005) Potential of telomerase expression and activity in cervical specimens as a diagnostic tool. J Clin Pathol 58:911–914
Trulsson LM, Velin AK, Herder A, Soderkvist P, Ruter A, Smeds S (2003) Telomerase activity in surgical specimens and fine-needle aspiration biopsies from hyperplastic and neoplastic human thyroid tissues. Am J Surg 186:83–88
Tomoda R, Seto M, Tsumuki H, Iida K, Yamazaki T, Sonoda J, Matsumine A, Uchida A (2002) Telomerase activity and human telomerase reverse transcriptase mRNA expression are correlated with clinical aggressiveness in soft tissue tumors. Cancer 95:1127–1233
Watanabe M, Yu SK, Sawafuji M, Kawamura M, Horinouchi H, Mukai M, Kobayashi K (2002) Enhanced expression of telomerase activity in thymoma and thymic carcinoma tissues: a clinicopathologic study. Cancer 94:240–244
Rudolph P, Schubert C, Tamm S, Heidorn K, Hauschild A, Michalska I, Majewski S, Krupp G, Jablonska S, Parwaresch R (2000) Telomerase activity in melanocytic lesions: a potential marker of tumor biology. Am J Pathol 156:1425–1432
Koyanagi K, Ozawa S, Ando N, Mukai M, Kitagawa Y, Ueda M, Kitajima M (2000) Telomerase activity as an indicator of malignant potential in iodine-nonreactive lesions of the esophagus. Cancer 88:1524–1529
Shoji Y, Yoshinaga K, Inoue A, Iwasaki A, Sugihara K (2000) Quantification of telomerase activity in sporadic colorectal carcinoma: association with tumor growth and venous invasion. Cancer 88:1304–1309
Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, Rischin D, Sauleda S, Gee J, Nicholson RI, Baselga J (2001) Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 20:110–124
Malik SH, Siu LL, Rowinsky EK, deGraffenried L, Hammond LA, Rizzo J, Bacus S, Brattain MG, Kreisberg JI, Hidalgo M (2003) Pharmacodynamic evaluation of the epidermal growth factor receptor inhibitor OSI-774 in human epidermis of cancer patients. Clin Cancer Res 9:2478–2486
Polychronis A, Sinnett HD, Hadjiminas D, Singhal H, Mansi JL, Shivapatham D, Shousha S, Jiang J, Peston D, Barrett N, Vigushin D, Morrison K, Beresford E, Ali S, Slade MJ, Coombes RC (2005) Preoperative gefitinib versus gefitinib and anastrazole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. Lancet Oncol 6:383–391
Parton M, Krajewski S, Smith I, Krajewska M, Archer C, Naito M, Ahern R, Reed J, Dowsett M (2002) Coordinate expression of apoptosis-associated proteins in human breast cancer before and during chemotherapy. Clin Cancer Res 8:2100–2108
Bergers E, Jannink I, van Diest PI, Cuesta MA, Meyer S, van Mourik JC, Baak JP (1997) The influence of fixation delay on mitotic activity and flow cytometric cell cycle variables. Human Pathol 23:95–100
Start RD, Cross SS, Clelland C, Silcocks PB, Rogers K, Smith JH (1992) Delay in fixation does not affect the immunoreactivity of proliferating cell nuclear antigen (PCNA). J Pathol 168:197–199
Start RD, Flynn MS, Cross SS, Rogers K, Smith JH (1991) Is the grading of breast carcinomas affected by a delay in fixation? Virchows Arch A Pathol Anat Histopathol 419:475–477
Cross SS, Start RD, Smith JH (1990) Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol 43:597
Graem N, Helweg-larsen K (1979) Mitotic activity and delay in fixation of tumour tissue. The influence of delay in fixation on mitotic activity of a human osteogenic sarcoma grown in athymic nude mice. Acta Pathol Microbiol Scand [A] 87A:375–378
Krupinski J, Slevin M, Marti E, Catena E, Rubio F, Gaffney J (2003) Time-course phosphorylation of the mitogen activated protein (MAP) kinase group of signalling proteins and related molecules following middle cerebral artery occlusion (MCAO) in rats. Neuropathol Appl Neurobiol 29:144–158
Skifter DA, Allegrini PR, Wiessner C, Mir AK (2002) Similar time-course of interleukin-1 beta production and extracellular-signal-related kinase (ERK) activation in permanent focal brain ischemic injury. Metab Brain Dis 17:131–138
Fincham VJ, Frame MC (1998) The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J 17:81–92
Young O, Jones RJ, Clack G, Jacobs V, Marshall A, Fennell M, Green T, Dixon JM: Src kinase activity in early breast cancer (EBC): a quantitative luminometric and immunohistochemical (IHC) approach. Proc Am Soc Clin Oncol 2006. Abstract 13010
Acknowledgements
Pamela de Clive Lowe (research nurse), Karina Meachin (study manager).
Competing interests
MF, VJ, ED, TG and GC are employees of AstraZeneca plc. RJ is a consultant to AstraZeneca plc.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work presented was supported by AstraZeneca plc.
Rights and permissions
About this article
Cite this article
Jones, R.J., Boyce, T., Fennell, M. et al. The impact of delay in cryo-fixation on biomarkers of Src tyrosine kinase activity in human breast and bladder cancers. Cancer Chemother Pharmacol 61, 23–32 (2008). https://doi.org/10.1007/s00280-007-0440-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0440-9