Abstract
Purpose
To develop a population pharmacokinetic (popPK) model for CP-724,714, a novel HER2 tyrosine kinase inhibitor is under development for the treatment of advanced HER2 positive cancers.
Methods
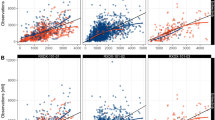
Concentration-time data (n = 484) from 30 cancer patients receiving daily oral CP-724,714 at doses of 250 mg QD, 250 mg BID, 250 mg TID, and 400 mg BID in 21-day cycles in an open label First-in-Human dose-escalation study were evaluated. Serum concentrations of CP-724,714 were obtained after single dose and at steady state. Nonlinear mixed effect analysis in NONMEM using first order conditional estimation with interaction (FOCEI) was performed. The effect of covariates was assessed. Diagnostic plots, decrease of objective function value (>7.8), bootstrapping, and predictive check were used as model selection criteria.
Results
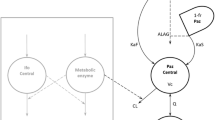
A 2-compartment first-order absorption pharmacokinetic (PK) model with inter-subject variability (ISV) on the apparent oral elimination clearance (CL/F), apparent central volume of distribution (V1/F), apparent peripheral volume of distribution (V2/F), and first-order oral absorption rate constant (ka), interoccasion variability (IOV) on CL/F, and body weight (WT) as covariate on CL/F was developed. There was no evidence of dose-dependent and/or time-dependent PK. CL/F increased with increasing WT. The ISV of CL/F was reduced by approximately 20% with WT as a covariate. Age, race, and liver metastasis did not influence CP-724,714 disposition.
Conclusions
A popPK model was developed that adequately described the pharmacokinetics of CP-724,714. WT was identified as a covariate on CL/F that reduced ISV and improved model performance. Future studies will further assess the importance of WT as a pharmacokinetic covariate. The proposed popPK model can be used to simulate CP-724,714 Phase 2/3 trials.





Similar content being viewed by others
References
Slamon DJ, Clark GM (1988) Amplification of c-erbB-2 and aggressive human breast tumors. Science 240:1795–1798
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Baselga J, Tripathy D, Mendelsohn J et al (1999) Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol 26:78–83
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719–726
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Seidman A, Hudis C, Pierri MK et al (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20:1215–1221
Jani JP, Barbacci G, Bhattacharya S, Boos C, Campbell M, Clark T, Coleman K, Currier N et al (2004) CP-724714, a novel erbB2 receptor tyrosine kinase inhibitor for cancer therapy. Proc Am Assoc Cancer Res 45:1071
Guo F, Letrent S, Noe D, Qin A, Rohrbacher K, Munster PN, Tolcher AW, Britten CD, Gelmon K, Sharma A (2006) Pharmacokinetics and pharmacodynamics of a HER2 tyrosine kinase inhibitor CP-724,714 in patients with advanced malignant HER2 positive solid tumors. Clin Pharmacol Ther 79:P10
Munster PN, Britten CD, Mita M, Gelmon K, Minton SE, Moulder S, Slamon DJ, Guo F, Letrent SP, Denis L, Tolcher AW (2006) First-in-human study of the safety and tolerability and pharmacokinetics of CP-724,714 in patients with advanced malignant solid tumors that express HER2. Clin Cancer Res (in press)
Bruno R, Hille D, Riva A, Vivier N, Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas FR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Van Kesteren C, Mathot RA, Raymond E, Armand JP, Dittrich C, Dumez H, Roche H, Droz JP, Punt C, Ravic M, Wanders J, Beijnen JH, Fumoleau P, Schellens JH (2002) Population pharmacokinetics of the novel anticancer agent E7070 during four phase I studies: model building and validation. J Clin Oncol 20:4065–4073
Aarons L, Karlsson MO, Mentre F, Rombout F, Steimer JL, van Peer A (2001) Role of modeling and simulation in Phase I drug development. Eur J Pharm Sci 13:115–122
Williams PJ, Ette EI (2000) The role of population pharmacokinetics in drug development in light of the Food and Drug Administration’s “Guidance for Industry: population pharmacokinetics”. Clin Pharmacokinet 39:385–395
Beal SL, Sheiner LB (1998) NONMEM user’s guide: NONMEM project group. University of California, San Francisco
Karlsson MO, Sheiner LB (1993) The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21:735–750
Ette EI (1997) Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37:486–495
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28:171–192
Holford NH (2005) The visual predictive check—superiority to standard diagnostic plots, the 2005 Population Approach Group In Europe (PAGE) annual meeting, Abstr 738, Pamplona, Spain
Parke J, Holford NH, Charles BG (1999) A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 59:19–29
Gibiansky E, Gibiansky L, Enriquez J (2005) Population pharmacokinetic model of sedative doses of GPI 15715 and propofol liberated from GPI 15715, the 2005 ASCPT annual meeting, Abstr PI-154, Orlando
Klein CE, Gupta E, Reid JM, Atherton PJ, Sloan JA, Pitot HC, Ratain MJ, Kastrissios H (2002) Population pharmacokinetic model for irinontecan and two of its metabolites, SN-38 and SN-38 glucuronide. Clin Pharmacol Ther 72:638–647
Launay-Iliadis MC, Bruno R, Cosson V, Vergniol JC, Oulid-Aissa D, Marty M, Clavel M, Aapro M, LeBail N, Iliadis (1995) A population pharmacokinetics of docetaxel during phase 1 studies using nonlinear mixed-effect modeling and nonparameteric maximum-likelihood estimation. Cancer Chemother Pharmacol 37:47–54
Chen XY, Bies RR, Ramanathan RK, Zuhowski EG, Ttrmp DL, Egorin MJ (2005) Population pharmacokinetic analysis of 17-(allylamino)-17-demethoxygeldanamycin (17AAG) in adult patients with advance malignancies. Cancer Chemother Pharmacol 55:237–243
Sheiner LB, Ludden TM (1992) Population pharmacokinetics/dynamics. Annu Rev Pharmacol Toxicol 32:185–209
Ette CI, Williams PJ (2004) Population pharmacokinetics II: estimation methods. Ann Pharmacother 38:1907–1915
Ribbing J, Jonsson EN (2004) Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J Pharmacokinet Pharmacodyn 1:109–134
Acknowledgment
The authors would like to acknowledge Dr. Dennis Noe, Dr. Diane Mould, Dr. Marc R. Gastonguay, and Dr. Steve Riley for valuable discussions in the preparation of the manuscript, Dr. Michael Avery and Mr. David Wolford for the analytical assay of CP-724,714, and the patients and members of the Pfizer CP-724,714 team for their contributions to the Phase 1 study. The authors also would like to warmly thank Dr. Louis Denis for his contributions in the Phase 1 trial design and execution, and Dr. Pamela N. Munster, Dr. Carolyn D. Britten, Dr. Monica Mita, Dr. Karen Gelmon, Dr. Susan E. Minton, Dr. Stacy Moulder, Dr. Dennis J. Slamon, and Dr. Anthony W. Tolcher for conducting this Phase 1 trial.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, F., Letrent, S.P. & Sharma, A. Population pharmacokinetics of a HER2 tyrosine kinase inhibitor CP-724,714 in patients with advanced malignant HER2 positive solid tumors. Cancer Chemother Pharmacol 60, 799–809 (2007). https://doi.org/10.1007/s00280-007-0427-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0427-6