Abstract
Purpose
The comparison between sonodynamic antitumor effect with protoporphyrin IX (PPIX) and hematoporphyrin (Hp) at a concentration of 5 mg/kg on Sarcoma 180 (S180) cells was studied in vivo, and the potential cell damage mechanism was also investigated.
Methods
The sonodynamically induced anti-tumor effect of PPIX was studied in mice bearing S180 solid tumors. In order to determine the optimum timing of ultrasound exposure after administration of PPIX, the PPIX concentrations in plasma, skin, muscle and tumor were determined by the fluorescence intensity of tissue extractions with a fluorescence spectrophotometer based on the standard curve. Anti-tumor effects were estimated by measuring the tumor size and the tumor weight. Additionally, the morphological changes of S180 cells were evaluated by transmission electron microscope (TEM) observation immediately after sonodynamic therapy (SDT) treatment.
Results
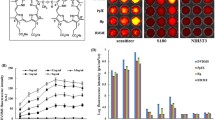
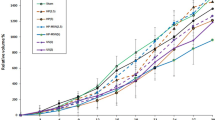
A time of 24 h after the intravenous administration of PPIX was chosen as the best time for ultrasound exposure. The antitumor effect induced by PPIX mediated sonodynamic therapy (PPIX-SDT) was in a dose dependent manner when ultrasound intensity was at or above the inertial cavitation threshold (5 W/cm2). A significant tumor growth delay was observed both in PPIX mediated sonodynamic therapy and in Hp mediated sonodynamic therapy treatments (Hp-SDT), and the tumor weight inhibition ratios after the synergistic treatments were 42.82 ± 0.03 and 35.22 ± 0.03%, respectively, this difference was significant at P < 0.05. While ultrasound alone (5 W/cm2) showed a slight tumor growth inhibitory effect compared with the control group, and PPIX or Hp alone showed almost no significant effect. Furthermore, TEM observation indicated cell damage was more serious in PPIX-SDT treatment group than in Hp-SDT treatment group. After sonication, the cell ultra-structure such as cell membrane destruction, mitochondria swelling, chromatin condensation might be important factors that inhibited the tumor growth and even induced cell death.
Conclusions
The comparative results suggested that PPIX as a sonosensitizer might have more potential cytotoxicity than Hp when irradiated with ultrasound, and the ultra-structural changes may account for cell destruction induced by sonodynamic therapy in our experiment mode.










Similar content being viewed by others
Reference
Umemura S, Yumita N, Nishifaki R, Umemura K (1989) Sonochemical activation of hematoporphyrin: a potential modality for cancer treatment. IEEE Ultrason Symp 9:955
Tachibana K, Kimura N, Okumura M, Eguchi H, Tachibana S (1993) Enhancement of cell killing of HL-60 cells by ultrasound in the presence of the photosensitizing drug Photofrin II. Cancer Lett 72:195
Kessel D, Jeffers R, Fowlkes JB, Cain C (1994) Porphyrin-induced enhancement of ultrasound cytotoxicity. Int J Radiat Biol 66:221
Kondo T, Umemura S, Tanabe K, Ogawa R, Adachi I, Riesz P (2000) Novel therapeutic applications of ultrasound: utilization of thermal and cavitational effects. Jpn J Hyperthermic Oncol 16:203
Feril LB, Kondo T, Umemura S, Tachibana K, Manalo AH, Riesz P (2002) Sound waves and antineoplastic drugs: the possibility of an enhanced combined anticancer therapy. J Med Ultrason 29:173
Rick K, Sroka R, Stepp H, Kriegmair M, Huber RM, Jacob K, Baumgartner R (1997) Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood. J Photochem Photobiol B 40:313
Kennedy JC, Pottier RH (1992) Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B 14:275
Sharwani A, Jerjes W, Salih V, MacRobert AJ, El-Maaytah M, Khalil HS, Hopper C (2006) Fluorescence spectroscopy combined with 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in detecting oral premalignancy. J Photochem Photobiol B 83:27
Abels C, FritschC, Bolsen K, Szeimies RM, Ruzicka T, Goerz G, Goetz AE (1997) Photodynamic therapy with 5-aminolaevulinic acid-induced porphyrins of an amelanotic melanoma in vivo. J Photochem Photobiol B 40:76
Fritsch C, Homey B, Stahl W, Lehmann P, Ruzicka T, Sies H (1998) Preferential relative porphyrin enrichment in solar keratoses upon topical application of delta-aminolevulinic acid methylester. Photochem Photobiol 68:218
Bartosova J, Hrkal Z (2000) Accumulation of protoporphyrin-IX (PpIX) in leukemic cell lines following induction by 5-aminolevulinic acid (ALA). Comp Biochem Physiol C Toxicol Pharmacol 126:245
Ji Z, Yang G, Vasovic V, Cunderlikova B, Suo Z, Nesland JM, Peng Q (2006) Subcellular localization pattern of protoporphyrin IX is an important determinant for its photodynamic efficiency of human carcinoma and normal cell lines. J Photochem Photobiol B 84:213
Lee CF, Lee CJ, Chen CT, Huang CT (2004) delta-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J Photochem Photobiol B 75:21
Schuitmaker JJ, Baas P, Leengoed HL, Meulen FW, Star WM, Zandwijk N (1996) Photodynamic therapy: a promising new modality for the treatment of cancer. J Photochem Photobiol B 34:3
Andreas Dietze, Kristian Berg (2005) ALA-induced porphyrin formation and fluorescence in synovitis tissue in vitro and in vivo studies. Photodiagn Photodyn Ther 4(2):299
Peng Q, Moan J, Warloe T, Nesland JM, Rimington C (1992) Distribution and photosensitizing efficiency of porphyrins induced by application of exogenous 5-aminolevulinic acid in mice bearing mammary carcinoma. Int J Cancer 52:433
Klinteberg C AF, Enejder AM, Wang I, Andersson-Engels S, Svanberg S, Svanberg K (1999) Kinetic fluorescence studies of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation in basal cell carcinomas. J Photochem Photobiol B 49:120
Orenstein A, Kostenich G, Malik Z (1997) The kinetics of protoporphyrin fluorescence during ALA-PDT in human malignant skin tumors. Cancer Lett 120:229
Kinoshita M, Hynynen K (2006) Mechanism of Porphyrin-Induced Sonodynamic Effect: Possible Role of Hyperthermia. Radiat Res 165:299
Liu QH, Wang XB, Wang P, Qi H, Zhang K, Xiao LN (2006) Sonodynamic effects of protoporphyrin IX disodium salt on isolated sarcoma 180 cells. Ultrasonics 45:56
Umemura S, Kawabata K, Sasaki K, Yumita N, Umemura K, Nishigaki R (1996) Recent advances in sonodynamic approach to cancer therapy. Ultra Sonochem 3(3):187
Liu QH, Sun SH, Xiao YP, Qi H, Shang ZY, Zhang J P, Zhang JX, Ren YH, Li M, Li Q(2003a) Study of cell killing on S180 by different intensity ultrasound activate hematoporphyrin derivatives. Sci China Ser C 46(3):253
Meng JW, Wang XJ, Lin T, Ren XG, Pang HF, Zhou CN (1999) The study of protoporphyrin IX metabolism in cell proliferation using photoluminescence method. J Lumin 83–84:271
Yumita N, Nishigaki R, Umemura S (2000a) Sonodynamically induced antitumor effect of Photofrin II on colon 26 carcinoma. J Cancer Res Clin Oncol 126(10):601
Yumita N, Umemura S (2003) Sonodynamic therapy with photofrin II on AH130 solid tumor. Cancer Chemother Pharmacol 51(2):174
Yumita N, Umemura S (2004) Sonodynamic antitumour effect of chloroaluminum phthalocyanine tetrasulfonate on murine solid tumour. J Pharm Pharmacol 56:85
Dougherty TJ (1993) Photodynamic therapy. Photochem Photobiol 58(6):895
Kessel D (1986) Porphyrin-lipoprotein association as a factor in porphyrin localization. Cancer Lett 33(2):183
Tang W, Liu QH, Liu SY (2005) Determination of HpD distribution in tumor bearing mouse with fluorescence photometer. J Northwest Univ (Nat Sci Edn) 35:436
Doan N, Reher P, Meghji S, Harris M (1999) In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J Oral Maxillofac Surg 57:409
Liu QH, Wang P, Li M, Qi H, Shang ZY, Ren YH, Zhang K, Yao X (2003) Apopotosis of Ehrl ich ascites tumor cells by sonochemical-activated hematoporphyrin. Acta Zoologica Sinica 49:620
Liu QH, Sun SH, Xiao YP, Qi H, Zhang JX, Ren YH, Wang P (2004) Synergistic anti-tumor effect of ultrasound and hematoporphyrin on sarcoma180 cells with special reference to the changes of morphology and cytochrome oxidase activity of tumor cells. J Exp Clin Cancer Res 23(2):333
Yu T, Wang Z, Mason TJ (2004) A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem 11(2):95
Rosenthal I, Sostaric JZ, Riesz (2004) Sonodynamic therapy—a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem 11(6):349
Yumita N, Nishigaki R, Sakata I, Nakajima S, Umemura S (2000) Sonodynamically induced antitumor effect of 4-formyloximeethylidene-3-hydroxy-2-vinyl-deuterio-porphynyl(IX) -6–7-diaspartic acid (ATX-S10). Jpn J Cancer Res 91(2):255
Ogawa K, Tachibana K, Uchida T, Tai T, Yamashita N, Tsujita N, Miyauchi R (2001) High-resolution scanning electron microscopic evaluation of cell-membrane porosity by ultrasound. Med Electron Microsc 34:249
Briviba K, Klotz LO, Sies H (1997) Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems. Biol Chem 378:1259
Umemura S, Yumita N, Umemura K, Nishigaki R (1999) Sonodynamically induced effect of rose bengal on isolated sarcoma 180 cells. Cancer Chemother Pharmacol 43:389
Xu YM, Zhang Y, Yang YZ (1995) Character of photosensitive damage of PP, Hp and DHE to the structure of main-chain of protein. Chin J Light Scatter 7:163
Liu QH, Liu SY, Qi H, Wang P, Tang W, Zhang K, Dai L, Shi YC (2005) Preliminary study on the mechanism of apoptosis in Ehrl ich ascites tumor cells by sonochemical activated hematoporphyrin. Acta Zoologica Sinica 51:1073
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No.39870240 and No. 30270383) and the Excellent Doctor Innovation Project of Shaanxi Normal University. The authors also gratefully acknowledge Dr Jo Richardson graduated from University of Cambridge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Wang, X., Wang, P. et al. Comparison between sonodynamic effect with protoporphyrin IX and hematoporphyrin on sarcoma 180. Cancer Chemother Pharmacol 60, 671–680 (2007). https://doi.org/10.1007/s00280-006-0413-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0413-4