Abstract
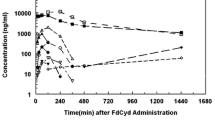
Objectives: The objectives of this study were to characterize pharmacokinetics of N-2-chloroethylaziridine (CEA) in the rat model and assess the in vivo fraction of total clearance of phosphoramide mustard (PM) that furnished CEA to circulation. Methods: The disposition of CEA was investigated following separate intravenous (iv) administrations of PM, synthetic CEA, and their combination to the Sprague-Dawley rats. In addition, in rats receiving prodrug cyclophosphamide (CP), plasma concentrations of CP and its metabolites, 4-hydroxycyclophosphamide (HOCP), PM, and CEA, were simultaneously quantified using GC/MS and stable isotope dilution techniques. Results: Following iv administration of synthetic CEA, concentrations of CEA declined biexponentially with the mean terminal half-life and total body clearance of 47.5 min and 167 ml/min/kg, respectively. Urinary excretion of unchanged CEA was 0.164% of the administered dose. CEA was found to be the major circulating metabolite after iv administration of precursor PM to rats. The fraction of total clearance of PM that furnished CEA to circulation was estimated to be 100%, indicating virtually complete availability of the metabolite to circulation once formed. In rats administered with CP, PM exhibited the highest plasma and urinary concentrations compared to HOCP and CEA. Conclusions: For the first time, CEA was demonstrated to be an important in vivo metabolite of CP in the present study. In light of the poor permeability and in vivo stability of PM, the ultimate DNA alkylator, the findings obtained in this study suggested that CEA may contribute significantly to the overall antitumor activity of prodrug CP.





Similar content being viewed by others
Abbreviations
- CP:
-
Cyclophosphamide
- HOCP:
-
4-hydroxycyclophosphamide
- PM:
-
Phosphoramide mustard
- CEA:
-
N-2-chloroethylaziridine
- NNM:
-
Nornitrogen mustard
References
Chen CS, Jounaidi Y, Waxman DJ (2005) Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expreesed cytochromes P450. Drug Metab Dispos 33:1261–1267
Philip PA, Ali-Sadat S, Doehmer J, Kocarek T, Akhtar A, Lu H, Chan KK (1999) Use of V79 cells with stably transfected cytochrome P450 cDNAs in studying the metabolism and effects of cytotoxic drugs. Cancer Chemoth Pharm 43:59–67
Brock N, Gross R, Hohorst HG, Klein HO, Schneider B (1971) Activation of cyclophosphamide in man and animals. Cancer 27:1512–1529
Hong PS, Srigritsanapol A, Chan KK (1991) Pharmacokinetics of 4-hydroxycyclophosphamide and metabolites in the rat. Drug Metab Dispos 19:1–7
Chan KK, Hong PS, Tutsch K, Trump DL (1994) Clinical pharmacokinetics of cyclophosphamide and metabolites with and without SR-2508. Cancer Res 54:6421–6429
Ludeman SM (1999) The chemistry of the metabolites of cyclophosphamide. Curr Pharm Design 5:627–643
Struck RF, Kirk MC, Witt MH, Laster RW (1975) Isolation and mass spectral identification of blood metabolites of cyclophosphamide: evidence for phosphoramide mustard as the biologically active metabolite. Biomed Environ Mass Spectrom 2:46–53
Colvin M, Brundrett RB, Kan MN, Jardin I, Fenselau C (1976) Akylating properties of phosphoramide mustard. Cancer Res 36:1122–1126
Chan KK, Zheng JJ, Wang JJ, Dea P, Muggia FM (1994) Retention of cytotoxicity of phosphoramide mustard (PM) aqueous solution after its complete degradation. Proc Am Assoc Cancer Res 35:300
Rauen HM, Norpoth K (1968) A volatile compound N-(2-chloroethyl) aziridine in the exhaled air of rats after administration of endoxan. Klin Wochenschr 46:272–275
Lu H, Chan KK (1996) Gas chromatographic-mass spectrometric assay for N-2-chloroethylaziridine, a volatile cytotoxic metabolite of cyclophosphamide, in rat plasma. J Chromatogr B 678:219–225
Flowers JL, Ludeman SM, Gamcsik MP, Colvin OM, Shao KL, Boal JH, Springer JB, Adams DJ (2000) Evidence for a role of chloroethylaziridine in the cytotoxicity of cyclophosphamide. Cancer Chemoth Pharm 45:335–344
Shulman-Roskes EM, Noe DA, Gamcsik MP, Marlow AL, Hilton J, Hausheer FH, Colvin OM, Ludeman SM (1998) The partitioning of phosphoramide mustard and its aziridinium ions among alkylation and P-N bond hydrolysis reactions. J Med Chem 41:515–529
Chan KK, Hong SC, Watson E, Deng SK (1986) Identification of new metabolites of phosphoraramide and nornitrogen mustards and cyclophosphamide in rat urine using ion cluster techniques. Biomed Mass Spectrom 13:145–154
Hong PS, Chan KK (1987) Identification and quantitation of alcophosphamide, a metabolite of cyclophosphamide, in the rat using chemical ionization mass spectrometry. Biomed Mass Spectrom 14:167–172
Watson E, Dea P, Chan KK (1985) Kinetics of phosphoramide mustard hydrolysis in aqueous solution. J Pharm Sci 74:1283–1292
Hong PS, Chan KK (1989) Analysis of 4-hydroxycyclophosphamide in plasma by gas chromatography-mass spectrometry. J Chromatogr 495:131–138
Wang JJ, Lu H, Chan KK (2000) Stereoselective pharmacokinetics of ifosfamide in male and female rats. AAPS Pharmsci, 2 (article 17):1–11
Mehvar R (1991) Relationship of apparent systemic clearance to individual organ clearances: Effect of pulmonary clearance and site of drug administration and measurement. Pharm Res 8(3):306–312
Yuan ZM, Smith PB, Brundrett RB, Colvin OM, C. Fenselau C (1991) Glutathione conjugation with phosphoramide mustard and cyclophosphamide-a mechanistic study using tandem mass spetrometry. Drug Metab Dispos 19:625–629
Millis KK, Colvin ME, Shulman-Roskes EM, Ludeman SM, Colvin OM, Gamcsik MP (1995) Comparison of the protonation of isophosphoramide and phosphoramide mustard. J Med Chem 38:2166–2175
Hata Y, Watanabe M (1994) Metabolism of aziridines and the mechanism of their cytotoxicity. Drug Metab Rev 26:575–604
Acknowledgements
This work was conducted in Kenneth Chan’s laboratory and supported by Biomedical Mass Spectrometry Laboratory, College of Pharmacy, The Ohio State University
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, H., Chan, K.K. Pharmacokinetics of N-2-chloroethylaziridine, a volatile cytotoxic metabolite of cyclophosphamide, in the rat. Cancer Chemother Pharmacol 58, 532–539 (2006). https://doi.org/10.1007/s00280-006-0196-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0196-7