Abstract
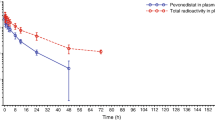
Purpose: To examine the antitumor activity and the pharmacokinetics of CPT-11 (irinotecan, 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxycamptothecin) in a plasma esterase-deficient scid mouse model, bearing human tumor xenografts. Experimental design: Plasma carboxylesterase (CE)-deficient mice were bred with scid animals to develop a strain that would allow growth of human tumor xenografts. Following xenotransplantation, the effect of the plasma esterase on antitumor activity following CPT-11 administration was assessed. In addition, detailed pharmacokinetic studies examining plasma and biliary disposition of CPT-11 and its metabolites were performed. Results: In mice lacking plasma carboxylesterase, the mean SN-38 systemic exposures were approximately fourfold less than that observed in control animals. Consistent with the pharmacokinetic data, four to fivefold more CPT-11 was required to induce regressions in human Rh30 xenografts grown in esterase-deficient scid mice, as opposed to those grown in scid animals. Additionally, the route of elimination of CPT-11, SN-38, and SN-38 glucuronide (SN-38G) was principally in the bile. Conclusions: The pharmacokinetic profile for CPT-11 and its metabolites in the esterase-deficient mice more closely reflects that seen in humans. Hence, these mice may represent a more accurate model for antitumor studies with this drug and other agents metabolized by CEs.






Similar content being viewed by others
Abbreviations
- AUC0-∞:
-
Area under the concentration-time curve from time 0 to infinity
- CE:
-
Carboxylesterase
- CL:
-
Clearance
- CPT-11:
-
Irinotecan, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin
- HPLC:
-
High-performance liquid chromatography
- o-NPA:
-
o-nitrophenyl acetate
- SN-38:
-
7-ethyl-10-hydroxycamptothecin
- SN-38G:
-
SN-38 glucuronide
- V c :
-
Volume of the central compartment
References
Beaufay H, Amar-Costesec A, Feytmans E, Thines-Sempoux D, Wibo M, Robbi M, Berthet J (1974) Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol 61:188–200
D-Argenio DZ, Schumitzky A (1979) A program package for simulation and parameter estimation in pharmacokinetic systems. Computer Prog Biomed 9:115–134
Danks MK, Morton CL, Krull EJ, Cheshire PJ, Richmond LB, Naeve CW, Pawlik CA, Houghton PJ, Potter PM (1999) Comparison of activation of CPT-11 by rabbit and human carboxylesterases for use in enzyme/prodrug therapy. Clin Cancer Res 5:917–924
Douglass EC, Valentine M, Etcubanas E, Parham D, Webber BL, Houghton PJ, Houghton JA, Green AA (1987) A specific chromosomal abnormality in rhabdomyosarcoma. Cytogenet Cell Genet 45:148–155
Furman WL, Stewart CF, Poquette CA, Pratt CB, Santana VM, Zamboni WC, Bowman LC, Ma MK, Hoffer FA, Meyer WH, Pappo AS, Walter AW, Houghton PJ (1999) Direct translation of a protracted irinotecan schedule from a xenograft model to a Phase I trial in children. J Clin Oncol 17:1815–1824
Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18:1095–1100
Guemei AA, Cottrell J, Band R, Hehman H, Prudhomme M, Pavlov MV, Grem JL, Ismail AS, Bowen D, Taylor RE, Takimoto CH (2001) Human plasma carboxylesterase and butyrylcholinesterase enzyme activity: correlations with SN-38 pharmacokinetics during a prolonged infusion of irinotecan. Cancer Chemother Pharmacol 47:283–290
Guichard S, Morton CL, Krull EJ, Stewart CF, Danks MK, Potter PM (1998) Conversion of the CPT-11 metabolite APC to SN-38 by rabbit liver carboxylesterase. Clin Cancer Res 4:3089–3094
Houghton PJ, Cheshire PJ, Hallman JD, 2nd, Lutz L, Friedman HS, Danks MK, Houghton JA (1995) Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol 36:393–403
Humerickhouse R, Lohrbach K, Li L, Bosron W, Dolan M (2000) Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res 60:1189–1192
Khanna R, Morton CL, Danks MK, Potter PM (2000) Proficient metabolism of CPT-11 by a human intestinal carboxylesterase. Cancer Res 60:4725–4728
Morton CL, Wierdl M, Oliver L, Ma M, Danks MK, Stewart CF, Eiseman JL, Potter PM (2000) Activation of CPT-11 in mice: Identification and analysis of a highly effective plasma esterase. Cancer Res 60:4206–4210
Morton CL, Taylor KR, Iacono L, Cheshire P, Houghton PJ, Danks MK, Stewart CF, Potter PM (2002) Metabolism of CPT-11 in esterase deficient mice. Proc Am Assoc Cancer Res 43:248
Potter PM, Pawlik CA, Morton CL, Naeve CW, Danks MK (1998) Isolation and partial characterization of a cDNA encoding a rabbit liver carboxylesterase that activates the prodrug Irinotecan (CPT-11). Cancer Res 52:2646–2651
Potter PM, Wolverton JS, Morton CL, Wierdl M, Danks MK (1998) Cellular localization domains of a rabbit and a human carboxylesterase: influence on irinotecan (CPT-11) metabolism by the rabbit enzyme. Cancer Res 58:3627–3632
Rivory LP, Haaz M-C, Canal P, Lokiec F, Armand J-P, Robert J (1997) Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in PhaseI/II trials. Clin Cancer Res 3:1261–1266
Satoh T, Hosokawa M, Atsumi R, Suzuki W, Hakusui H, Nagai E (1994) Metabolic activation of CPT-11, 7-ethyl-10-[4-(1-piperidino)-1- piperidino]carbonyloxycamptothecin, a novel antitumor agent, by carboxylesterase. Biol Pharm Bull 17:662–664
Soares ER (1979) Identification of a new allele of Es-1 segregating in an inbred strain of mice. Biochem Genet 17:577–583
Takasuna K, Kasai Y, Kitano Y, Mori K, Kakihata K, Hirohashi M, Nomura M (1995) Study on the mechanisms of diarrhea induced by a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Nippon Yakurigaku Zasshi 105:447–60
Tanizawa A, Fujimori A, Fujimori Y, Pommier Y (1994) Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst 86:836–842
Wadkins RM, Potter PM, Vladu B, Marty J, Mangold G, Weitman S, Manikumar G, Wani MC, Wall ME, Von Hoff DD (1999) Water soluble 20(S)-glycinate esters of 10,11-methylenedioxycamptothecins are highly active against human breast cancer xenografts. Cancer Res 59:3424–3428
Wierdl M, Morton CL, Weeks JK, Danks MK, Harris LC, Potter PM (2001) Sensitization of human tumor cells to CPT-11 via adenoviral-mediated delivery of a rabbit liver carboxylesterase. Cancer Res 61:5078–5082
Acknowledgments
We thank Dr. J. P. McGovren for the gifts of CPT-11 and SN-38, and Yakult Honsha for SN-38G. This work was supported in part by NIH grants CA76202, CA79763, CA23099, the Cancer Center Core Grant CA21765 and the American Lebanese Syrian Associated Charities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morton, C.L., Iacono, L., Hyatt, J.L. et al. Activation and antitumor activity of CPT-11 in plasma esterase-deficient mice. Cancer Chemother Pharmacol 56, 629–636 (2005). https://doi.org/10.1007/s00280-005-1027-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-1027-y