Abstract
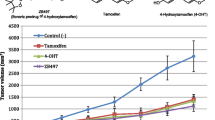
Objectives: To determine the effect of tamoxifen on the pharmacokinetics of a single 250 mg oral dose of gefitinib (IRESSA) in healthy volunteers. Methods: An open-label, single-center, phase I study in healthy male volunteers. Each volunteer received a single 250 mg oral dose of gefitinib on day 1. On days 11–14, oral loading doses of 60 mg tamoxifen were administered, followed by 20 mg tamoxifen for a further 16 days to maintain steady-state exposure. On day 24, volunteers received a second single 250 mg oral dose of gefitinib. The last dose of tamoxifen was given on day 30. Pharmacokinetic and safety assessments were conducted throughout the trial. Results: A total of 18 volunteers were recruited. The presence of tamoxifen did not have a clinically significant effect on the primary variables AUC and Cmax of gefitinib, nor on the secondary variables AUC(0-t), tmax, t1/2, and λz. The geometric least square mean values for AUC were 3,407.6 versus 3,397.9 ng.h/ml in the absence and presence of tamoxifen, respectively (90% CL 0.894, 1.112) and for Cmax were 110.8 versus 103.6 ng/ml, respectively (90% CL 0.786, 1.111). The combination of gefitinib with tamoxifen was generally well tolerated by the volunteers. There were no serious adverse events and no volunteer discontinued the study due to an adverse event. NCI-CTC grade 1/2 drug-related adverse events were observed in seven volunteers, including loose stools and skin events associated with gefitinib, and lethargy and headache, flushing, and dizziness associated with tamoxifen. Conclusions: This study suggests that tamoxifen has no significant effect on the pharmacokinetics, tolerability, or safety of a single 250 mg oral dose of gefitinib. Therefore, in clinical investigations of this combination, no dose adjustment of gefitinib is indicated.



Similar content being viewed by others
References
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard J-Y, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong R-P, Baselga J (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 21:2237–2246
Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. A randomized trial. JAMA 290:2149–2158
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Rose C, Mouridsen HT (1984) Treatment of advanced breast cancer with tamoxifen. Recent Results Cancer Res 91:230–242
Gee JMW, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, McClelland RA, Jordan N, Wakeling AE, Nicholson RI (2003) The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology 144:5105–5117
Adam HK, Patterson JS, Kemp JV (1980) Studies on the metabolism and pharmacokinetics of tamoxifen in normal volunteers. Cancer Treat Rep 64:761–764
Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR (2002) Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos 30:608–612
Dowsett M, Pfister C, Johnston SRD, Miles DW, Houston SJ, Verbeek JA, Gundacker H, Sioufi A, Smith IE (1999) Impact of tamoxifen on the pharmacokinetics and endocrine effects of the aromatase inhibitor letrozole in postmenopausal women with breast cancer. Clin Cancer Res 5:2338–2343
The ATAC Trialists’ Group (2001) Pharmacokinetics of anastrozole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the ‘Arimidex and tamoxifen alone or in combination’ (ATAC) trial. Br J Cancer 85:317–324
Food and Drug Administration (2004) Guidance for industry. Statistical approaches to establishing bioequivalence. Available at: http://www.fda.gov/cder/guidance/guidance.htm
Tischkowitz MD, Hodgson SV, Fentiman IS (2002) Male breast cancer: aetiology, genetics and clinical management. Int J Clin Pract 56:750–754
Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA (1999) Tamoxifen versus placebo in the treatment of Peyronie’s disease. J Urol 162:2003–2005
Clarke SC, Schofield PM, Grace AA, Metcalfe JC, Kirschenlohr HL (2001) Tamoxifen effects on endothelial function and cardiovascular risk factors in men with advanced atherosclerosis. Circulation 103:1497–1502
Wilkinson PM, Ribiero GG, Adam HK, Kemp JV, Patterson JS (1982) Tamoxifen (Nolvadex) therapy - rationale for loading dose followed by maintenance dose for patients with metastatic breast cancer. Cancer Chemother Pharmacol 10:33–35
Bradford DA, Mant TG, Amin DA, Angell MP (2003) The placebo effect: adverse events commonly seen in healthy volunteer phase I drug trials—an observation on findings, comparing active with placebo treatment (abstract no. OII-C-4). Clin Pharmacol Ther 73:P92
Author information
Authors and Affiliations
Corresponding author
Additional information
IRESSA is a trademark of the AstraZeneca group of companies
Rights and permissions
About this article
Cite this article
Cantarini, M.V., Macpherson, M.P., Marshall, A.L. et al. A phase I study to determine the effect of tamoxifen on the pharmacokinetics of a single 250 mg oral dose of gefitinib (IRESSA) in healthy male volunteers. Cancer Chemother Pharmacol 56, 557–562 (2005). https://doi.org/10.1007/s00280-005-1007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-1007-2