Abstract
Purpose
The aim of this study was to investigate the therapeutic value and safety of third-line treatment with mitomycin-C (MMC) and capecitabine (Xeloda) in patients with advanced colorectal cancer pretreated with combination regimens including 5-fluorouracil (5-FU), folinic acid (FA) and irinotecan (CPT-11) or 5-FU, FA and oxaliplatin (L-OHP).
Patients and methods
A total of 21 patients (M/F 16/5, median age 60.0 years) with advanced colorectal cancer, all of whom had developed progressive disease while receiving or within 6 months of discontinuing two sequential chemotherapy lines with 5-FU, FA and CPT-11 or 5-FU, FA and L-OHP, were accrued to this study. At the time of their relapse or progression, cytotoxic chemotherapy, consisting of intravenous MMC 7 mg/m2 on therapeutic day 1 plus oral capecitabine 1000 mg/m2 twice daily on days 1–14, was initiated. After rest for 7 days, capecitabine 1000 mg/m2 twice daily was administered on days 22–35 followed by 7 days rest. Treatment courses were repeated every 6 weeks unless there was evidence of progressive disease, unacceptable toxicity or patient refusal of treatment.
Results
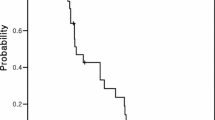
All the patients were assessable for toxicity and 19 for response. The median number cycles of chemotherapy was two (range one to four). Only 1 patient (4.8%) had a partial response, 4 patients (19.0%) had stable disease, and 14 patients (66.7%) progressed. The median follow-up period was 7.3 months and median time to progression was 2.6 months. The median overall survival was 6.8 months. No toxic deaths occurred. Toxicities of third-line treatment were mild and manageable. As NCI/NIH common toxicity criteria, grade 3/4 anemia, neutropenia and thrombocytopenia occurred in two, one and one patients, respectively.
Conclusion
Our findings suggest that the combination of MMC and capecitabine in patients with advanced colorectal cancer pretreated with combination regimens including 5-FU, FA and CPT-11 or 5-FU, FA and L-OHP is safe. However, this regimen had a poor response rate and no definitive contribution to increasing patients’ overall survival time. Further evaluation of other salvage regimens seems to be warranted.


Similar content being viewed by others
References
Meta-analysis Group In Cancer (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301–308
Kohne C, Van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz M, Lorenz M, Anak O, Genicot B (2003) Irinotecan improves the activity of the AIO regimen in metastatic colorectal cancer: Results of EORTC GI Group study 40986. Proc Am Soc Clin Oncol 22:254
Grothey A, Schmoll HJ (2001) New chemotherapy approaches in colorectal cancer. Curr Opin Oncol 13:275–286
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041–1047
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Chester JD, Dent JT, Wilson G, Ride E, Seymour MT (2000) Protracted infusional 5-fluorouracil (5-FU) with bolus mitomycin in 5-FU-resistant colorectal cancer. Ann Oncol 11:235–237
Seitz JF, Perrier H, Giovannini M, Capodano G, Bernardini D, Bardou VJ (1998) 5-Fluorouracil, high-dose folinic acid and mitomycin C combination chemotherapy in previously treated patients with advanced colorectal carcinoma. J Chemother 10:258–265
Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H (1998) Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol 55:1091–1097
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Kondo Y, Terashima M, Sato A, Taguchi T (2004) A pilot phase II study of capecitabine in advanced or recurrent colorectal cancer. Jpn J Clin Oncol 34:195–201
Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B (2000) Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 45:291–297
Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H (1998) Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res 4:1013–1019
Ross P, Norman A, Cunningham D, Webb A, Iveson T, Padhani A, Prendiville J, Watson M, Massey A, Popescu R, Oates J (1997) A prospective randomised trial of protracted venous infusion 5-fluorouracil with or without mitomycin C in advanced colorectal cancer. Ann Oncol 8:995–1001
Rosati G, Rossi A, Germano D, Reggiardo G, Manzione L (2003) Raltitrexed and mitomycin-C as third-line chemotherapy for colorectal cancer after combination regimens including 5-fluorouracil, irinotecan and oxaliplatin: a phase II study. Anticancer Res 23:2981–2985
Hoff PM, Pazdur R, Lassere Y, Carter S, Samid D, Polito D, Abbruzzese JL (2004) Phase II study of capecitabine in patients with fluorouracil-resistant metastatic colorectal carcinoma. J Clin Oncol 22:2078–2083
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214
Icli F, Arican A, Cay F, Akbulut H, Dincol D, Karaoguz H, Demirkazik A (1999) Phase II study of cisplatin and dacarbazine for metastatic colorectal carcinoma resistant to 5-fluorouracil. Oncology 56:297–300
Zorzitto ML, Myers R, Bazos MJ, Shepherd FA, Evans WK (1986) Methyl-CCNU and methotrexate therapy in patients with advanced colorectal cancer after failure of 5-fluorouracil chemotherapy. Am J Clin Oncol 9:27–30
Adenis A, Carlier D, Darloy F, Pion JM, Bonneterre J, Demaille A (1995) Cytarabine and cisplatin as salvage therapy in patients with metastatic colorectal cancer who failed 5-fluorouracil + folinic acid regimen. French Northern Oncology Group. Am J Clin Oncol 18:158–160
Tsavaris N, Kosmas C, Gennatas K, Vadiaka M, Skopelitis E, Xila V, Rokana S, Margaris E, Zografos G, Papastratis G, Kouraklis G (2002) Etoposide, leucovorin (LV) and 5-fluorouracil (5-FU) in 5-FU + LV pre-treated patients with advanced colorectal cancer. J Chemother 14:406–411
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 22:337–345
Kikuyama S, Inada T, Oyama R, Ogata Y (2002) Phase II study of mitomycin C, cisplatin and 5-fluorouracil for advanced and recurrent gastric cancer. Anticancer Res 22:3633–3636
Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, Depisch D, Lang F, Scheithauer W (2004) Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol 15:478–483
Hofheinz RD, Weisser A, Willer A, Hehlmann R, Hochhaus A (2003) Treatment of a patient with advanced esophageal cancer with a combination of mitomycin C and capecitabine: activation of the thymidine phosphorylase as active principle? Onkologie 26:161–164
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, D.H., Park, Y.S., Park, BB. et al. Mitomycin-C and capecitabine as third-line chemotherapy in patients with advanced colorectal cancer: a phase II study. Cancer Chemother Pharmacol 56, 10–14 (2005). https://doi.org/10.1007/s00280-004-0963-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0963-2