Abstract
Purpose
Capecitabine is a three-step prodrug that was rationally designed to be a more effective and safer alternative to its intermediate metabolite, 5′-deoxy-5-fluorouridine (5′-DFUR). We compared the pharmacokinetics/pharmacodynamics of these drugs in metastatic breast cancer patients.
Methods
Six patients received oral capecitabine at 1657 mg/m2 twice daily and 17 received 5′-DFUR at 400 mg three times daily. Both drugs were administered for 21 days followed by a 7-day rest.
Results
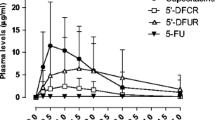
Median daily 5′-DFUR AUC was significantly higher for capecitabine than for 5′-DFUR (81.1 vs 32.6 mmol h/l; P=0.01). Following treatment with 5′-DFUR, the median AUC and Cmax of 5′-DFUR tended to be higher in patients with a partial response (3.83 μg h/ml and 4.88 μg/ml) and stable disease (6.46 μg h/ml and 4.96 μg/ml) than in those with disease progression (2.53 μg h/ml and 1.36 μg/ml). The AUC and Cmax of 5′-DFUR was significantly related to overall survival.
Conclusions
These results support the superiority of capecitabine over 5′-DFUR.

Similar content being viewed by others
References
Alberto P, Winkelmann JJ, Paschoud N, Peytremann R, Bruyere A, Righetti A, Decoster G, Holdener EE (1989) Phase I study of oral doxifluridine using two schedules. Eur J Cancer Clin Oncol 25:905–908
Bajetta E, Colleoni M, Di Bartolomeo M, Buzzoni R, Bozzetti F, Doci R, Somma L, Cappuzzo F, Stampino CG, Guenzi A, et al (1995) Doxifluridine and leucovorin: an oral treatment combination in advanced colorectal cancer. J Clin Oncol 13:2613–2619
Blesch KS, Gieschke R, Tsukamoto Y, Reigner BG, Burger HU, Steimer JL (2003) Clinical pharmacokinetic/pharmacodynamic and physiologically based pharmacokinetic modeling in new drug development: the capecitabine experience. Invest New Drugs 21:195–223
Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17:485–493
Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, Behr J, Gordon RJ, Osterwalder B, Griffin T (1998) Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 16:1795–1802
Eda H, Fujimoto K, Watanabe S, Ura M, Hino A, Tanaka Y, Wada K, Ishitsuka H (1993) Cytokines induce thymidine phosphorylase expression in tumor cells and make them more susceptible to 5′-deoxy-5-fluorouridine. Cancer Chemother Pharmacol 32:333–338
Etienne MC, Chatelut E, Pivot X, Lavit M, Pujol A, Canal P, Milano G (1998) Co-variables influencing 5-fluorouracil clearance during continuous venous infusion. A NONMEM analysis. Eur J Cancer 34:92–97
Gieschke R, Burger HU, Reigner B, Blesch KS, Steimer JL (2003) Population pharmacokinetics and concentration–effect relationships of capecitabine metabolites in colorectal cancer patients. Br J Clin Pharmacol 55:252–263
Howell A, Mackintosh J, Jones M, Redford J, Wagstaff J, Sellwood RA (1988) The definition of the ‘no change’ category in patients treated with endocrine therapy and chemotherapy for advanced carcinoma of the breast. Eur J Cancer Clin Oncol 24:1567–1572
Ishikawa T, Sekiguchi F, Fukase Y, Sawada N, Ishitsuka H (1998) Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase activities in tumors in human cancer xenografts. Cancer Res 58:685–690
Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H (1998) Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol 55:1091–1097
Ishitsuka H (2000) Capecitabine: preclinical pharmacology studies. Invest New Drugs 18:343–354
Judson IR, Beale PJ, Trigo JM, Aherne W, Crompton T, Jones D, Bush E, Reigner B (1999) A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14C-labelled drug. Invest New Drugs 17:49–56
Mackean M, Planting A, Twelves C, Schellens J, Allman D, Osterwalder B, Reigner B, Griffin T, Kaye S, Verweij J (1998) Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 16:2977–2985
McLeod HL, Sludden J, Hardy SC, Lock RE, Hawksworth GM, Cassidy J (1998) Autoregulation of 5-fluorouracil metabolism. Eur J Cancer 34:1623–1627
Milano G, Etienne MC, Renee N, Thyss A, Schneider M, Ramaioli A, Demard F (1994) Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol 12:1291–1295
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Nakao I, Saito T, Kimura K, Wakui A, Yokoyama M, Kanamaru R, Furue H, Komita T, Ohta K, Murakami M, et al (1985) Phase I study of 5′-deoxy-5-fluorouridine (5′-DFUR). Gan To Kagaku Ryoho 12:2037–2043
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40:85–104
Reigner B, Verweij J, Dirix L, Cassidy J, Twelves C, Allman D, Weidekamm E, Roos B, Banken L, Utoh M, Osterwalder B (1998) Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res 4:941–948
Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B (2000) Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 45:291–297
Suzuki S, Hongu Y, Fukazawa H, Ichihara S, Shimizu H (1980) Tissue distribution of 5′-deoxy-5-fluorouridine and derived 5-fluorouracil in tumor-bearing mice and rats. Gann 71:238–245
Tominaga T, Koyama H, Toge T, Miura S, Sugimachi K, Yamaguchi S, Hirata K, Monden Y, Nomura Y, Toi M, Kimijima I, Noguchi S, Sonoo H, Asaishi K, Ikeda T, Morimoto T, Ota J, Ohashi Y, Abe O (2003) Randomized controlled trial comparing oral doxifluridine plus oral cyclophosphamide with doxifluridine alone in women with node-positive breast cancer after primary surgery. J Clin Oncol 21:991–998
Twelves C, Glynne-Jones R, Cassidy J, Schuller J, Goggin T, Roos B, Banken L, Utoh M, Weidekamm E, Reigner B (1999) Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res 5:1696–1702
Van Der Heyden SA, Highley MS, De Bruijn EA, Tjaden UR, Reeuwijk HJ, Van Slooten H, Van Oosterom AT, Maes RA (1999) Pharmacokinetics and bioavailability of oral 5′-deoxy-5-fluorouridine in cancer patients. Br J Clin Pharmacol 47:351–356
Zampino MG, Colleoni M, Bajetta E, Stampino CG, Guenzi A, de Braud F (1999) Pharmacokinetics of oral doxifluridine in patients with colorectal cancer. Tumori 85:47–50
Zhang J, Mizoi T, Harada N, Shiiba K, Miyagawa K, Matsuno S, Sasaki I (2003) Thymidine phosphorylase expressed in macrophages enhances antitumor effect of 5′-deoxy-5-fluorouridine on human colorectal carcinoma cells. Anticancer Res 23:323–329
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebi, H., Sigeoka, Y., Saeki, T. et al. Pharmacokinetic and pharmacodynamic comparison of fluoropyrimidine derivatives, capecitabine and 5′-deoxy-5-fluorouridine (5′-DFUR). Cancer Chemother Pharmacol 56, 205–211 (2005). https://doi.org/10.1007/s00280-004-0934-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0934-7