Abstract
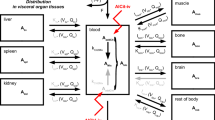
Parenteral administration of arsenic trioxide has recently been recognized as an effective antineoplastic therapy, especially for the treatment of acute promyelocytic leukemia. Its efficacy and toxicity are concentration-dependent and are related to the fractions of different arsenic species and the degree of methylation. In this study, arsenic trioxide was given parenterally to rabbits as a single dose or as a daily dose (0.2, 0.6, and 1.5 mg/kg) for 30 days. The blood and organ concentrations of the arsenic species, including As(III), dimethylarsinic acid (DMA), and monomethylarsonic acid (MMA), were studied on day 1 (single-dose study), day 30 (multiple dosing study), and day 60 (reversibility study). As(III) was the major detectable arsenic species in the blood. The pharmacokinetic parameters (total clearance, area under the curve, etc.) for As(III) indicated a limit for the capacity to eliminate As(III) at the dose of 1.5 mg/kg, and were quite the same after a single dose or chronic multiple dosing. In tissues, DMA was found to be the major metabolite and the concentrations of DMA, As(III), and MMA in general increased with the dose, with the increase most significant at a dose of 1.5 mg/kg. However, normalized tissue distribution of As(III) in the kidney on day 1, but not on day 30, was nonlinear. Along with decreased levels of As(III) and increased levels of DMA, an inducible capacity for methylating As(III) to DMA after chronic dosing in kidney was suggested. The tissue concentration of DMA was highest in lung and liver, and the normalized tissue distributions in liver on day 30 were nonlinear, suggesting a limit in eliminating DMA after a chronic high load of As(III). Tissue concentrations of As(III), DMA, and MMA in bladder increased dramatically after chronic dosing. However, after washout for 30 days, As(III), DMA, and MMA were all undetectable in bladder and liver. However, As(III) in hair and low levels of DMA in lung, kidney, heart and hair were still detected. In conclusion, in rabbits we found a similar pharmacological profile after a single dose or chronic multiple dosing of parenteral arsenic trioxide, with a limiting metabolizing capacity at a dose of 1.5 mg/kg. Tissue accumulation of arsenic species, mainly DMA, and its reversibility after washout were tissue-selective. The potential for late toxicities of arsenic trioxide in organs with a significant tendency for arsenic accumulation with low reversibility should be closely monitored.


Similar content being viewed by others
References
Squibb KS, Fowler BA (1983) The toxicity of arsenic and its compounds. In: Fowler BA (ed) Biological and environmental effects of arsenic. Elsevier, Amsterdam, pp 233–235
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang Z (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL). II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89:3354–3360
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XH, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Zhu C (1996) In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 apoptosis with downregulation of Bc1-2 expression and modulation of PML-RARα/PML proteins. Blood 88:1052–1061
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL). I. As2O3 exerts dose-dependent dual effect on APL cells. Blood 89:3345–3353
Huang SY, Chang CS, Tang JL, Tien HF, Kuo TL, Huang SF, Yao YT, Chou WC, Chung CY, Wang CH, Shen MC, Chen YC (1998) Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukemia. Br J Haematol 103:1092–1095
Huang CH, Chen WJ, Wu CC, Chen YC, Lee YT (1999) Complete atrioventricular block after arsenic trioxide treatment in an acute promyelocytic leukemic patient. Pacing Clin Electrophysiol 22:965–967
Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, Wu W, Zhang FQ, Chen Y, Zhou L, Li JM, Zeng XY, Yang RR, Yuan MM, Ren MY, Gu FY, Cao Q, Gu BW, Su XY, Chen GQ, Xiong SM, Zhang T, Waxman S, Wang ZY, Chen Z, Hu J, Shen ZX, Chen SJ (1999) Studies on treatment of acute promyelocytic leukemia with arsenic trioxide. Remission induction, follow-up and molecular monitoring in 11 newly diagnosed and 47 relapsed APL patients. Blood 94:3315–3324
Ohnish K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Natio K, Shinjo K, Fujita Y (2000) Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med 133:881–885
Unnikrishnan D, Dutcher JP, Lucariello R, Api M, Garl S, Wiernik PH, Chiaramida S (2001) Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood 97:1514–1516
Marafante E, Bertolero F, Edel J, Pietra R, Sabbioni E (1982) Intracellular interaction and biotransformation of arsenite in rats and rabbits. Sci Total Environ 24:27–39
Yamauchi H, Yamamura Y (1985) Metabolism and excretion of orally administrated arsenic trioxide in the hamster. Toxicology 34:113–121
Yamanaka K, Hoshino M, Okamoto M, Sawamuara R, Hasegawa A, Okada S (1990) Induction of DNA damage by dimethylarsine, a metabolite of inorganic arsenics, is for the major part likely due to its peroxyl radical. Biochem Biophys Res Commun 168:58–64
Murai T, Iwata H, Otoshi T, Endo G, Horiguchi S, Fukushima S (1993) Renal lesions induced in F344/DuCrj rats by 4-weeks oral administration of dimethylarsonic acid. Toxicol Lett 66:53–61
Thompson DJ (1993) A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact 88:89–114
Yamanaka K, Ohtsubo K, Hasegawa A, Haashi H, Ohji H, Kanisawa M, Okada S (1996) Exposure to dimethylarsonic acid, a main metabolite of inorganic arsenics strongly promotes tumorigenesis initiated by 4-nitroquinoline 1-oxide in the lungs of mice. Carcinogenesis 17:767–770
Styblo M, Serves SV, Cullen WR, Thomas DJ (1997) Comparative inhibition of yeast glutathione reductase by arsenicals and arseothiols. Chem Res Toxicol 10:27–33
Li W, Wanibuchi H, Salim EI, Yamamoto S, Yoshida K, Endo G, Fukushima S (1998) Promotion of NCI-Black_Reiter male rat bladder carcinogenesis by dimethylarsonic acid an organic arsenic compound. Cancer Lett 134:29–36
Marafante E, Rade J, Sabbioni E (1981) Intracellular interaction and metabolic fate of arsenite in the rabbit. Clin Toxicol 18:1335–1341
Vahter M, Marafante E (1983) Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chem Biol Interact 47:29–44
Maiorino RM, Aposhian HV (1985) Dimercaptan metal-binding agents influence the biotransformation of arsenite in the rabbit. Toxicol Appl Pharmacol 77:240–250
Bogdan GM, Sampayo-Reyes A, Aposhian HV (1994) Arsenic binding proteins of mammalian systems: isolation of three arsenite-binding proteins of rabbit liver. Toxicology 93:175–193
Gomez-Ariza JL, Sanchez-Rodas I, Giraldez I, Morales E (2000) Comparison of biota sample pretreatments for arsenic speciation with coupled HPLC-HG-ICP-MS. Analyst 125:401–407
Hsueh YM, Huang YL, Huang CC, Wu WL, Chen HM, Yang MH, Lue LC, Chen CJ (1998) Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health 54:431–444
Vahter M (1999) Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog 82:69–88
Chang WC, Chen SH, Wu HL, Shi GY, Murata SI, Morita I (1991) Cytoprotective effect of reduced glutathione in arsenical-induced endothelial cell injury. Toxicology 69:101–110
Huang H, Huang CF, Wu DR, Jinn CM, Jan KY (1993) Glutathione as a cellular defense against arsenite toxicity in cultured Chinese hamster ovary cells. Toxicology 79:195–204
Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP (2001) Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol 60:302–309
Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW (2000) The MRP2/cMOAT transporter and arsenic–glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem 275:33404–33408
Wu MH, Lin CJ, Chen CL, Su MJ, Sun SS, Cheng AL (2003) Direct cardiac effects of As2O3 in rabbits: evidence of reversible chronic toxicity and tissue accumulation of arsenicals after parenteral administration. Toxicol Appl Pharmacol 189:214–220
Romach EH, Zhao CQ, Razo LMD, Cebrian ME, Waalkes MP (2000) Studies on the mechanisms of arsenic-induced self tolerance developed in liver epithelial cells through continuous low-level arsenite exposure. Toxicol Sci 54:500–508
Steinmaus C, Yuan Y, Bates MN, Smith AH (2003) Case-control study of bladder cancer and drinking water arsenic in the western United States. Am J Epidemiol 158:1193–1201
Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, Chen CJ (2001) Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol 153:411–418
Bode AM, Dong Z (2002) The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol 42:5–24
Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M (1996) The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J 15:6269–6279
Simeonova PP, Wang S, Toriumi W, Kommineni C, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI (2000) Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with AP-1 transactivation. Cancer Res 60:3445–3453
Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 4:289–299
Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Aposhian V (2000) Monomethylarsonous acid is more toxic than arsenite in chang human hepatocytes. Toxicol Appl Pharmacol 163:203–207
Petrick JS, Jagadish B, Mash EA, Aposhian HV (2001) Monomethylarsonous acid and arsenite: LD50 in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol 14:651–656
Yamanaka K, Hayashi H, Kato K, Hasegawa A, Okada S (1995) Involvement of preferential formation of apurinic/apyrimidinic sites in dimethylarsonic-induced DNA strand breaks and DNA-protein cross-links in cultured alveolar epithelial cell. Biochem Biophys Res Commun 207:244–249
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, CJ., Wu, MH., Hsueh, YM. et al. Tissue distribution of arsenic species in rabbits after single and multiple parenteral administration of arsenic trioxide: tissue accumulation and the reversibility after washout are tissue-selective. Cancer Chemother Pharmacol 55, 170–178 (2005). https://doi.org/10.1007/s00280-004-0872-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0872-4