Abstract
Purpose
To clarify the role of the pathways dependent on protein-kinase C (PK-C) and Ca2+/calmodulin (CaM) in the regulation of telomerase activity in Burkitt’s lymphoma cells.
Methods
Burkitt’s lymphoma cells (Raji and Daudi) were treated with the PK-C inhibitor, bisindolylmaleimide (BIM), or the CaM inhibitor, trifluoperazine (TFPZ), in a dose-dependent manner and in a time-dependent manner. The activities of PK-C isoenzymes were analyzed fluorimetrically using POLARIS assay kits. CaM-kinase II activity was analyzed radiographically, using CaMK-II immunoprecipitation kinase assay kits. Telomerase activity was detected by a conventional telomeric repeat amplification protocol and Stretch PCR. The level of catalytic subunit of telomerase (hTERT) in drug-treated and nontreated cells was analyzed by flow cytometry using anti-hTERT antibody labeled with ZenonAlexa Fluor-488 IgG. Apoptosis was estimated in terms of phosphatidylserine exposure on the cell surface and DNA fragmentation.
Results
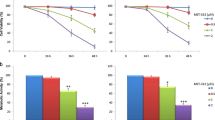
It was found that BIM inhibited telomerase activity and this process preceded apoptosis. The subsequent addition of exogenous PK-C (mixture of isoenzymes) to the cell lysates restored telomerase activity if incubation of cells with BIM was up to 24 h. Using PK-C isoenzymes, it was established that atypical PK-Cζ, but not conventional Ca2+-dependent PK-Cα, PK-Cβ or PK-Cγ, is responsible for the reactivation of telomerase in BIM-treated cells. BIM also showed a well-expressed cytotoxicity against intact leukemia cells. In contrast, the CaM inhibitor TFPZ showed the same cytotoxic effect without any influence on telomerase activity during incubation for 24 h with leukemia cells. After incubation for 48 h, TFPZ markedly suppressed telomerase activity. However, the effect followed apoptosis and appeared to be a result of cell death. The addition of exogenous CaMK-II to the cell lysates obtained from TFPZ-treated cells did not reactivate telomerase.
Conclusion
The present study confirmed the participation of atypical PK-Cζ, but not conventional Ca2+-dependent PK-C isoenzymes (α, β, γ) nor the Ca2+/CaM-dependent pathway, in the regulation of telomerase activity in Burkitt’s lymphoma cells.






Similar content being viewed by others
References
Blackburn EH (1992) Telomerase. Annu Rev Biochem 61:113
De Lange T (1994) Activation of telomerase in human tumors. Proc Natl Acad Sci USA 91:2882
De Lange T (2002) Protection of mammalian telomeres. Oncogene 21:532
Zakian VA (1995) Telomeres: beginning to understand the end. Science 270:1601
Greider CW (1996) Telomere length regulation. Annu Rev Biochem 65:337
McCaul JA, Gordon KE, Clark LJ, Parkinson EK (2002) Telomerase inhibition and the future management of head-and-neck cancer. Lancet Oncol 3:280
Kelland LR (2001) Telomerase: biology and phase I trials. Lancet Oncol 2:95
Sheng WY, Chien YL, Wang TC (2003) The dual role of protein kinase C in the regulation of telomerase activity in human lymphocytes. FEBS Lett 540:91
Bodnar AG, Kim NW, Effros RB, Chiu CP (1996) Mechanism of telomerase induction during T cell activation. Exp Cell Res 228:58
Gauthier LR, Granotier C, Soria JC, Faivre S, Boige V, Raymond E, Boussin FD (2001) Detection of circulating carcinoma cells by telomerase activity. Br J Cancer 84:631
Kyo S, Inoue M (2002) Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene 21:688
Liu JP (1999) Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J 13:2091
Antal M, Boros E, Solymosy F, Kiss T (2002) Analysis of the structure of human telomerase RNA in vivo. Nucleic Acids Res 30:912
Lavelle F, Riou JF, Laoui A, Mailliet P (2000) Telomerase: a therapeutic target for the third millennium? Crit Rev Oncol Hematol 34:111
Chang JT, Chen YL, Yang HT, Chen CY, Cheng AJ (2002) Differential regulation of telomerase activity by six telomerase subunits. Eur J Biochem 269:3442
Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Dickinson Caddle S, Ziaugra L, Beijersbergen RL, Davidoff ML, et al (1997) hEST2, the putative human telomerase catalytic subunit gene, is upregulated in tumor cells and during immortalization. Cell 90:785
Harrington L, McPhail T, Mar V, Zhou W, Oulten R, Bass MB, Arruda I, Robinson MO (1997) A mammalian telomerase-associated protein. Science 275:973
Mitchel JR, Wood E, Collins K (1999) Functional requirement of p23 and Hsp90 in telomerase complexes. Nature 402:551
Liu JP (1997) Protein phosphorylation events in exocytosis and endocytosis. Clin Exp Pharmacol Physiol 24:611
Kang SS, Kwon T, Kwon DY, Do SI (1999) Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem 274:13085
Li H, Zhao LL, Funder JW, Liu J-P (1997) Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem 272:16729
Alfonso-De Matte MY, Yang H, Evans MS, Cheng JQ, Kruk PA (2002) Telomerase is regulated by c-Jun NH2-terminal kinase in ovarian surface epithelial cells. Cancer Res 62:4575
Li H, Zhao LL, Funder JW, Liu J-P (1998) Telomerase is controlled by protein kinase Cα in human breast cancer cells. J Biol Chem 273:33436
Ku WC, Cheng AJ, Wang TC (1997) Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture. Biochem Biophys Res Commun 241:730
Yu C-C, Lo S-C, Wang T-C (2001) Telomerase is regulated by protein kinase C-ζ in human nasopharyngeal cancer cells. Biochem J 355:459
Kharbanda S, Kumar V, Dhar S, Pandey P, Chen C, Majumder P, Yuan ZM, Whang Y, Strauss W, Pandita TK, Weaver D, Kufe D (2000) Regulation of the hTERT telomerase catalytic subunit by c-Abl tyrosine kinase. Curr Biol 10:568
Maida Y, Kyo S, Kanaya T, Wang Z, Yatabe N, Tanaka M, Nakamura M, Ohmichi M, Gotoh N, Murakami S, Inoue M (2002) Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene 21:4071
Alfonso-De Matte MY, Moses-Soto H, Kruk PA (2002) Calcium-mediated telomerase activity in ovarian epithelial cells. Arch Biochem Biophys 399:239
Gutierrez-Cruz G, Van Heerden AH, Wang K (2001) Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J Biol Chem 276:7442
Igarashi H, Sakaguchi N (1997) Telomerase activity is induced in human peripheral B lymphocytes by the stimulation to antigen receptor. Blood 89:1299
Parmer TG, Ward MD, Yurkow EJ, Vyas VH, Kearney TJ, Hait WN (1999) Activity and regulation by growth factors of calmodulin-dependent protein kinase III (elongation factor 2-kinase) in human breast cancer. Br J Cancer 79:59
Nilsson A, Nygard O (1995) Phosphorylation of eukaryotic elongation factor 2 in differentiating and proliferating HL-60 cells. Biochim Biophys Acta 268:263
Nairn AC, Picciotto MR (1994) Calcium/calmodulin-dependent protein kinases. Semin Cancer Biol 5:295
Crouch SP, Kozlovsky R, Slater KJ, Fletcher J (1993) The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods 160:81
Ioannou YA, Chen FW (1996) Quantitation of DNA fragmentation in apoptosis. Nucleic Acids Res 24:992
Cheng EH, Gorelick FS, Czernik AJ, Bagaglio DM, Hait WH (1995) Calmodulin-dependent protein kinases in rat glioblastoma. Cell Growth Differ 6:615
Kaptein JS, Lin CK, Wang CL, Nguyen TT, Kalunta CI, Park E, Chen FS, Lad PM (1996) Anti-IgM-mediated regulation of c-myc and its possible relationship to apoptosis. J Biol Chem 271:18875
Sharp NA, Luscombe MJ, Clemens MJ (1989) Regulation of c-fgr proto-oncogene expression in Burkitt’s lymphoma cells: effect of interferon treatment and relationship to EBV status and c-myc mRNA levels. Oncogene 4:1043
Mathas S, Rickers A, Bommert K, Dorken B, Mapara MY (2000) Anti-CD20-and B-cell receptor-mediated apoptosis: evidence for shared intracellular signaling pathways. Cancer Res 60:7170
Poole JC, Andrews LG, Tollefsbol TO (2001) Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT). Gene 269:1
Goekjian PG, Jirousek MR (1996) Protein kinase C in the treatment of disease: signal transduction pathway, inhibitors, and agents in development. Curr Med Chem 6:877
Kiss Z, Deli E, Girard PR, Pettit GR, Kuo JF (1987) Comparative effects of polymyxin B, phorbol ester and bryostatin on protein phosphorylation, protein kinase C translocation, phospholipid metabolism and differentiation of HL60 cells. Biochem Biophys Res Commun 146:208
Kim YW, Hur SY, Kim TE, Lee JM, Namkoong SE, Ki IK, Kim JW (2001) Protein kinase C modulates telomerase activity in human cancer cells. Exp Mol Med 33:156
Pelassy C, Breittmayer J-P, Aussel C (2000) Regulation of phosphatidylserine exposure at the cell surface by the serine−base exchange enzyme system during CD95-induced apoptosis. Biochem Pharmacol 59:855
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods 184:39
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415
Tait JF, Gibson D (1992) Phospholipid binding of annexin V: effects of calcium and membrane phosphatidylserine content. Arch Biochem Biophys 298:187
Chacon-Cruz E, Oelberg DG, Davis P, Buescher ES (1998) Membrane depolarization and depletion of intracellular calcium stores are associated with delay of apoptosis in human neutrophils. J Leukoc Biol 64:759
Wood JP, Osborne NN (1997) Induction of apoptosis in cultured human retinal pigmented epithelial cells: the effect of protein kinase C activation and inhibition. Neurochem Int 31:261
Karahashi H, Nagata K, Ishii K, Amano F (2000) A selective inhibitor of p38 MAP kinase, SB202190, induced apoptotic cell death of a lipopolysaccharide-treated macrophage-like cell line, J774.1. Biochim Biophys Acta 1502:207
Lucas M, Sanchez-Margalet V, Sanz A, Solano F (1994) Protein kinase C activation promotes cell survival in mature lymphocytes prone to apoptosis. Biochem Pharmacol 47:667
Acknowledgements
The technical assistance of Mrs. Kawahara of the National Institute of Advanced Industrial Science and Technology—AIST-Kyushu, Japan, is gratefully acknowledged. This study was supported in part by the JSPS Invitation Fellowship Program for Research in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakalova, R., Ohba, H., Zhelev, Z. et al. Atypical protein-kinase Cζ, but neither conventional Ca2+-dependent protein-kinase C isoenzymes nor Ca2+-calmodulin, participates in regulation of telomerase activity in Burkitt’s lymphoma cells. Cancer Chemother Pharmacol 54, 161–172 (2004). https://doi.org/10.1007/s00280-004-0789-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0789-y