Abstract
Purpose
To determine the maximum tolerated dose and dose-limiting toxicities (DLTs) of ZD9331 in combination with cisplatin in patients with refractory solid tumors and to describe any preliminary antitumor activity associated with this regimen.
Materials and methods
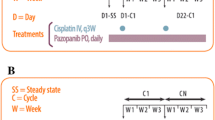
Patients received combination therapy with ZD9331 as a 30-min infusion on days 1 and 8 of a 21-day cycle at doses of 100 or 130 mg/m2, followed by cisplatin at 50 or 75 mg/m2 as a 30- to 60-min infusion on day 1 only.
Results
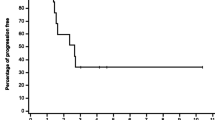
A total of 16 patients received 59 cycles of ZD9331 and cisplatin. Patients were enrolled at three dose levels: ZD9331/cisplatin 100/50 (n=3), 130/50 (n=9), 130/75 (n=4). DLTs at 130/75 included thrombocytopenia, neutropenia, fatigue, nausea, vomiting and stomatitis. Among 15 evaluable patients, 2 showed a partial response (patients with mesothelioma and head and neck cancer) and 6 showed stable disease (for a median of 5.5 cycles).
Conclusions
ZD9331 in combination with cisplatin was well tolerated at a dose of 130/50 mg/m2 after establishing the principal DLTs of neutropenia and thrombocytopenia. The combination shows evidence of antitumor activity in a pretreated population.
Similar content being viewed by others
References
Ayusawa D, Arai H, Wataya Y, Seno T (1988) A specialized form of chromosomal DNA degradation induced by thymidylate stress in mouse FM3A cells. Mutat Res 200:221
Cripps C, Burnell M, Jolivet J, Batist G, Lofters W, Dancey J, Iglesias J, Fisher B, Eisenhauer EA (1999) Phase II study of first-line LY231514 (multi-targeted antifolate) in patients with locally advanced or metastatic colorectal cancer: an NCIC Clinical Trials Group study. Ann Oncol 10:1175
Danenberg PV, Malli H, Swenson S (1999) Thymidylate synthase inhibitors. Semin Oncol 26:621
de Jonge MJ, Punt CJ, Sparreboom A, Planting AS, Peters ME, van De Schraaf J, Jackman A, Smith R, de Mulder PH, Verweij J (2002) Phase I and pharmacologic study of oral ZD9331, a novel nonpolyglutamated thymidylate synthase inhibitor, in adult patients with solid tumors. J Clin Oncol 20:1923
Diab S, Britten C, Smith R, et al (1998) A phase I pharmacokinetic study of ZD9331, a novel long-acting thymidylate synthase inhibitor on a single dosing every 3 weeks schedule (abstract 611). 10th NCI-EORTC Symposium on New Drugs in Cancer Therapy, Amsterdam, p 160
Eastman A (1987) The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther 34:155
Goh BC, Ratain MJ, Bertucci D, Smith R, Mani S, Vogelzang NJ, Schilsky RL, Hutchison M, Smith M, Averbuch S, Douglass E (2001) Phase I study of ZD9331 on short daily intravenous bolus infusion for 5 days every 3 weeks with fixed dosing recommendations. J Clin Oncol 19:1476
Ingraham HA, Dickey L, Goulian M (1986) DNA fragmentation and cytotoxicity from increased cellular deoxyuridylate. Biochemistry 25:3225
Jackman AL, Aherne GW, Kimbell, et al (1994) ZD9331, a non-polyglutamatable quinazoline thymidylate synthase (TS) inhibitor (abstract 1791). Proc Am Assoc Cancer Res 35:301
Jackman AL, Kelland LR, Kimbell R, Brown M, Gibson W, Aherne GW, Hardcastle A, Boyle FT (1995) Mechanisms of acquired resistance to the quinazoline thymidylate synthase inhibitor ZD1694 (Tomudex) in one mouse and three human cell lines. Br J Cancer 71:914
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649
Plummer R, Rees C, Judson I, et al (1999) Phase I trial of ZD9331 in adult patients with refractory solid malignancies administered by 30-minute infusion on days 1 and 8 with the cycle repeated every 3 weeks. Eur J Cancer 35:S285
Rees C, Beale P, Trigo JM, Mitchell F, Jackman A, Smith R, Douglass E, Judson I (2003) Phase I trial of ZD9331, a nonpolyglutamatable thymidylate synthase inhibitor, given as a 5-day continuous infusion to patients with refractory solid malignancies. Clin Cancer Res 9:2049
Rusthoven JJ, Eisenhauer E, Butts C, Gregg R, Dancey J, Fisher B, Iglesias J (1999) Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small-cell lung cancer: a phase II study. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 17:1194
Scanlon KJ, Lu Y, Kashani-Sabet M, Ma J, Newman E (1988) Mechanisms for cisplatin-FUra synergism and cisplatin resistance in human ovarian carcinoma cells both in vitro and in vivo. Adv Exp Med Biol 244:127
Trigo J, Rees C, Beale P, et al (1999) Phase I trial of ZD9331, a non-polyglutamatable thymidylate synthase inhibitor given as a 5-day continuous infusion every 3 weeks. Eur J Cancer 35:S286
Welsh SJ, Titley J, Brunton L, Valenti M, Monaghan P, Jackman AL, Aherne GW (2000) Comparison of thymidylate synthase (TS) protein up-regulation after exposure to TS inhibitors in normal and tumor cell lines and tissues. Clin Cancer Res 6:2538
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant from AstraZeneca.
Rights and permissions
About this article
Cite this article
Bilenker, J.H., Stevenson, J.P., Flaherty, K.T. et al. Phase I trial of the antifolate ZD9331 in combination with cisplatin in patients with refractory solid malignancies. Cancer Chemother Pharmacol 53, 357–360 (2004). https://doi.org/10.1007/s00280-003-0735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0735-4