Abstract
Purpose
The majority of patients who initially respond to trastuzumab will progress within 1 year. Currently, patients who progress after trastuzumab-based therapy are often maintained on trastuzumab combined with a different chemotherapeutic agent, such as vinorelbine. However, evidence supporting the continued use of trastuzumab in these breast cancers is lacking.
Methods
We created a preclinical model of trastuzumab resistance using the SKBR3 HER-2-overexpressing breast cancer cell line. Dose-response and cell cycle alterations in response to trastuzumab and/or vinorelbine were assessed.
Results
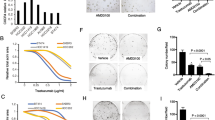
In contrast to the parental SKBR3 cells, vinorelbine-mediated growth inhibition and apoptosis were not significantly enhanced by the addition of trastuzumab in the trastuzumab-resistant pools.
Conclusions
These results suggest that the continued treatment of trastuzumab-resistant breast cancers with trastuzumab-containing regimens may not be effective. A randomized clinical trial of trastuzumab plus vinorelbine versus vinorelbine alone should be conducted in patients with HER-2-overexpressing breast cancer to determine the optimal duration of trastuzumab therapy upon progression.


Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177
Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L (1996) Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 14:737
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2 overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17:2639
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783
Seidman AD, Fornier M, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D’Andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, Norton L, Hudis C (2001) Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 19:2587
Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, Sneige N, Smith TL, Hortobagyi GN (2002) Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 20:1800
Esteva FJ, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN (2001) Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist 6:133
Pegram MD, Lopez A, Konecny G, Slamon DJ (2000) Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin Oncol 27:5
Burstein HJ, Kuter I, Campos S.M, Gelman RS, Tribou L, Parker LM, Manola J, Younger J, Matulonis U, Bunnell CA, Partridge AH, Richardson PG, Clarke K, Shulman LN, Winer EP (2001) Clinical activity of trastuzumab and vinorelbine in women with HER-2 overexpressing metastatic breast cancer. J Clin Oncol 19:2722
Burstein HJ, Harris LN, Marcom PK, Lambert-Falls R, Havlin K, Overmoyer B, Friedlander RJ, Gargiulo J, Strenger R, Vogel CL, Ryan PD, Ellis MJ, Nunes RA, Bunnell CA, Campos SM, Hallor M, Gelman R, Winer EP (2003) Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol 21:2889
Jahanzeb M, Mortimer JE, Yunus F, Irwin DH, Speyer J, Koletsky AJ, Klein P, Sabir T, Kronish L (2002) Phase II trial of weekly vinorelbine and trastuzumab as first-line therapy in patients with HER2(+) metastatic breast cancer. Oncologist 7:410
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27
Hahm HA, Dunn VR, Butash KA, Deveraux WL, Woster PM, Casero RA Jr, Davidson NE (2001) Combination of standard cytotoxic agents with polyamine analogues in the treatment of breast cancer cell lines. Clin Cancer Res 7:391
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719
Mackey J, Gelmon KA, Verma S, Gertler SZ, Bangemann N, Klimo P, Schneeweib A, Soulieres D, Bell R (2002) Continued use of Herceptin after disease progression in women with HER2 positive metastatic breast cancer: results from a retrospective analysis of 105 cases. Proc Am Soc Clin Oncol 21:207
Lu YH, Zi XL, Zhao YH, Mascarenhas D, Pollak M (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93:1852
Albanell J, Baselga J (2001) Unraveling resistance to trastuzumab (Herceptin): insulin-like growth factor-I receptor, a new suspect. J Natl Cancer Inst 93:1830
Yu DH (2001) Mechanisms of ErbB2-mediated paclitaxel resistance and trastuzumab-mediated paclitaxel sensitization in ErbB2-overexpressing breast cancers. Semin Oncol 28:12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nahta, R., Esteva, F.J. In vitro effects of trastuzumab and vinorelbine in trastuzumab-resistant breast cancer cells. Cancer Chemother Pharmacol 53, 186–190 (2004). https://doi.org/10.1007/s00280-003-0728-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0728-3