Abstract
Aim
The aim of this phase II study was to determine the efficacy and tolerability of the bimonthly, pharmacokinetically intensified LV5FU2 regimen in the treatment of metastatic colorectal cancers.
Methods
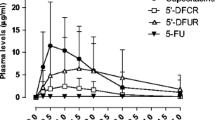
A total of 53 patients (23% second-line; 25 male/28 female; mean age 67 years; WHO performance status 0 in 38, 1 in 10 and 2 in 5) were treated in cycle 1 with the standard LV5FU2 regimen (leucovorin 200 mg/m2 per day followed by a 5-FU bolus 400 mg/m2 per day and a 22-h 5-FU continuous infusion 600 mg/m2 per day for two consecutive days every 2 weeks), and the AUC in mg·h/l·m2 was calculated. For cycle 2, according to a predefined schedule depending on the cycle-1 AUC value, in the absence of grade 3 toxicity, the 5-FU infusion dose was increased by 150% for AUC ≤5, by 100% for AUC >5–10, by 50% for AUC >10–15, and by 25% for AUC >15–20. 5-FU plasma concentrations were determined using high-performance liquid chromatography. A Bayesian methodology was used to assess individual pharmacokinetic parameters using the NONMEM computer program.
Results
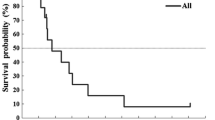
Among the 53 eligible patients, 87% (per-protocol population) received an increased dose in cycle 2 and 72% received the same dose. The median relative dose intensity was 1.28 (range 0.5–1.54) compared with the non-adapted theoretical total 5-FU dose. The objective response rate was 37% (95% CI 23–50%) in the intention-to-treat population and 47% (95% CI 29–65%) in the first-line per-protocol population. The median response duration was 10.4 months. The median progression-free survival (PFS) and overall survival (OS) were, respectively, 7 and 18.6 months. PFS and OS in first-line per-protocol patients were, respectively, 9.2 and 20 months. No deaths were attributed to toxicity of 5-FU despite the high doses administered. Of the 53 patients, 19% experienced gastrointestinal and 30% haematological grade 3/4 toxicities. Hand-foot syndrome was common but mild (grade 3 in one patient).
Conclusions
This strategy could be compared in a phase III trial with the standard LV5FU2 regimen.


Similar content being viewed by others
References
Advanced Colorectal Cancer Meta-Analysis Project (1992) Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 10:896
Andre T, Louvet C, Raymond E, Tournigand C, de Gramont A (1998) Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol 9:1251
Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17:3560
Ardalan B, Chua L, Tian EM, Reddy R, Sridhar K, Benedetto P, Richman S, Legaspi A, Waldman S, Morrell L, et al (1991) A phase II study of weekly 24-hour infusion with high-dose fluorouracil with leucovorin in colorectal carcinoma. J Clin Oncol 9:625
Beal SL (1992) NONMEM user's guide. University of California at San Francisco
Bressolle F, Joulia JM, Pinguet F, Ychou M, Astre C, Duffour J, Gomeni R (1999) Circadian rhythm of 5-fluorouracil population pharmacokinetics in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 44:295
de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15:808
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041
Erlichman C, Fine S, Elhakim T (1986) Plasma pharmacokinetics of 5-FU given by continuous infusion with allopurinol. Cancer Treat Rep 70:903
Fety R, Rolland F, Barberi-Heyob M, Merlin JL, Conroy T, Hardouin A, Riviere A, Milano G (1994) Clinical randomized study of 5FU monitoring versus standard dose in patients with head and neck cancer: preliminary results. Anticancer Res 14:2347
Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH, Gesta PH, Larra FG (1996) Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 77:441
Gamelin E, Boisdron-Celle M, Delva R, Regimbeau C, Cailleux PE, Alleaume C, Maillet ML, Goudier MJ, Sire M, Person-Joly MC, Maigre M, Maillart P, Fety R, Burtin P, Lortholary A, Dumesnil Y, Picon L, Geslin J, Gesta P, Danquechin-Dorval E, Larra F, Robert J (1998) Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 16:1470
Gamelin E, Jacob J, Danquechin-dorval E, Pezet D, Delva R, Raoul J (1998) Multicentric randomized trial comparing in weekly treatment of advanced colorectal cancer (CRC) intensified 5-fluorouracil and folinic acid (FA) with 5-FU pharmacokinetic monitoring to a constant dose calculated with body surface area (Meeting abstract). American Society of Clinical Oncology, Alexandria, VA, p 270
Hillcoat BL, McCulloch PB, Figueredo AT, Ehsan MH, Rosenfeld JM (1978) Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br J Cancer 38:719
Hryniuk WM, Figueredo A, Goodyear M (1987) Applications of dose intensity to problems in chemotherapy of breast and colorectal cancer. Semin Oncol 14:3
Joulia JM, Pinguet F, Ychou M, Duffour J, Topart D, Grosse PY, Astre C, Bressolle F (1997) Pharmacokinetics of 5-fluorouracil (5-FUra) in patients with metastatic colorectal cancer receiving 5-FUra bolus plus continuous infusion with high dose folinic acid (LV5FU2). Anticancer Res 17:2727
Kohne CH, Schoffski P, Wilke H, Kaufer C, Andreesen R, Ohl U, Klaasen U, Westerhausen M, Hiddemann W, Schott G, Harstick A, Bade J, Horster A, Schubert U, Hecker H, Dorken B, Schmoll HJ (1998) Effective biomodulation by leucovorin of high-dose infusion fluorouracil given as a weekly 24-hour infusion: results of a randomized trial in patients with advanced colorectal cancer. J Clin Oncol 16:418
Levi F, Giacchetti S, Adam R, Zidani R, Metzger G, Misset JL (1995) Chronomodulation of chemotherapy against metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Eur J Cancer 31A:1264
Levi F, Soussan A, Adam R, Caussanel JP, Metzger G, Jasmin C, Bismuth H, Smolensky M, Misset JL (1995) A phase I-II trial of five-day continuous intravenous infusion of 5- fluorouracil delivered at circadian rhythm modulated rate in patients with metastatic colorectal cancer. J Infus Chemother 5:153
Levi F, Zidani R, Misset JL (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350:681
Milano G, Roman P, Khater R, Frenay M, Renee N, Namer M (1988) Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer 41:537
Milano G, Etienne MC, Cassuto-Viguier E, Thyss A, Santini J, Frenay M, Renee N, Schneider M, Demard F (1992) Influence of sex and age on fluorouracil clearance. J Clin Oncol 10:1171
Poon MA, O'Connell MJ, Wieand HS, Krook JE, Gerstner JB, Tschetter LK, Levitt R, Kardinal CG, Mailliard JA (1991) Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol 9:1967
Santini J, Milano G, Thyss A, Renee N, Viens P, Ayela P, Schneider M, Demard F (1989) 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 59:287
Shuster JJ (1991) Median follow-up in clinical trials. J Clin Oncol 9:191
Spicer DV, Ardalan B, Daniels JR, Silberman H, Johnson K (1988) Reevaluation of the maximum tolerated dose of continuous venous infusion of 5-fluorouracil with pharmacokinetics. Cancer Res 48:459
Thyss A, Milano G, Renee N, Vallicioni J, Schneider M, Demard F (1986) Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother Pharmacol 16:64
Tournigand C, A de Gramont, Louvet C, Andre T, Carola E, Gilles-Amar V, Maindrault-Goebel F, Lotz JP, Molitor JL, Izrael V, Ecstein-Fraisse E, Krulik M (1998) A simplified bi-monthly regimen with leucovorin (LV) and 5-fluorouracil (5FU) for metastatic colorectal cancer (MCRC) (Meeting abstract). American Society of Clinical Oncology, Alexandria, VA, p 274
Vokes EE, Ratain MJ, Mick R, McEvilly JM, Haraf D, Kozloff M, Hamasaki V, Weichselbaum RR, Panje WR, Wenig B, et al (1993) Cisplatin, fluorouracil, and leucovorin augmented by interferon alfa-2b in head and neck cancer: a clinical and pharmacologic analysis. J Clin Oncol 11:360
Ychou M, Duffour J, Pinguet F, Kramar A, Joulia JM, Topart D, Bressolle F (1999) Individual 5FU-dose adaptation schedule using bimonthly pharmacokinetically modulated LV5FU2 regimen: a feasibility study in patients with advanced colorectal cancer. Anticancer Res 19:2229
Acknowledgement
We thank the "Ligue contre le Cancer" for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ychou, M., Duffour, J., Kramar, A. et al. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol 52, 282–290 (2003). https://doi.org/10.1007/s00280-003-0658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0658-0