Abstract
Purpose
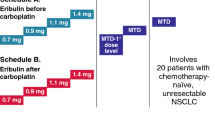
A phase I study was conducted to determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of carboplatin in combination with paclitaxel using a biweekly schedule in patients with advanced non-small-cell lung cancer (NSCLC).
Patients and methods
The pharmacokinetics of paclitaxel were determined preliminarily in some patients. The criteria for eligibility for study entry included histologically and/or cytologically confirmed NSCLC (stage IIIb or IV), no prior treatment, and measurable disease. Paclitaxel was given in combination with a fixed dose of carboplatin at an area under the concentration-time curve (AUC) of 3 mg/ml·min, every 2 weeks. The starting dose of paclitaxel was 100 mg/m2, and the dose was increased in increments of 20 mg/m2. Three to six patients were allocated to each dose level.
Results
A total of 19 patients (11 male and 8 female) with a median age of 61 years (range 43–74 years) and a median ECOG performance status of 0 (range 0–1) were enrolled. The MTD of paclitaxel proved to be 160 mg/m2, and the DLT was neutropenia, which improved well following treatment with G-CSF. Gastrointestinal toxicity was well tolerated. Of 17 patients who received four cycles or more, 7 (41%; 95% confidence interval 18.4–67.1%) responded to this combination therapy. The pharmacokinetics of paclitaxel did not differ from published data.
Conclusions
The recommended dose for phase II study is paclitaxel 140 mg/m2 with a carboplatin AUC of 3 mg/ml·min. This biweekly regimen is highly effective and acceptable, and the present data indicate that the regimen may be suitable for use on an outpatient basis.

Similar content being viewed by others
References
ASCO Special Article (1997) Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on 16 May 1997 by the American Society of Clinical Oncology. J Clin Oncol 15:2996–3018
Belani CP, Aisner J, Hiponia D, Ramanathan R (1996) Paclitaxel and carboplatin in metastatic non-small cell lung cancer: preliminary results of a phase I study. Semin Oncol 23 [5 Suppl 12]:19–21
Belani CP, Kearns CM, Zuhowski EG, Erkmen K, Hiponia D, Zacharski D, Engstrom C, Ramanathan RK, Capozzoli MJ, Aisner J, Egorin MJ (1999) Phase I trial, including pharmacokinetic and pharmacodynamic correlations, of combination paclitaxel and carboplatin in patients with metastatic non-small-cell lung cancer. J Clin Oncol 17:676–684
Briasoulis E, Kalofonos H, Bafaloukos D, Samantas E, Fountzilas G, Xiros N, Skarlos D, Christodoulou C, Kosmidis P, Pavlidis N (2000) Carboplatin plus paclitaxel in unknown primary carcinoma: a phase II Hellenic Cooperative Oncology Group study. J Clin Oncol 18:3101–3107
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
Evans WK, Earle CC, Stewart DJ, Dahrouge S, Tomiak E, Goss G, Logan D, Goel R, Gertler SZ, Dulude H (1997) Phase II study of a one hour paclitaxel infusion in combination with carboplatin for advanced non-small cell lung cancer. Lung Cancer 18:83–94
Glorieux P, Ortmanns P, Marien S, Degives R, Degraeve D, Potvin M, Grauwels D, Schallier D (2001) Multi-center study of two dose levels of paclitaxel with carboplatin in locally advanced and metastatic non-small cell lung cancer (NSCLC). Anticancer Res 21:1487–1494
Greco FA, Hainsworth JD (1998) One-hour paclitaxel plus carboplatin for advanced non-small-cell lung cancer. Oncology 12 [1 Suppl 2]:71–73
Grem JL, Tutsch KD, Simon KJ, Alberti DB, Willson JK, Tormey DC, Swaminathan S, Trump DL (1987) Phase I study of taxol administered as a short iv infusion daily for 5 days. Cancer Treat Rep 71:1179–1184
Hainsworth JD, Urba WJ, Hon JK, Thompson KA, Stagg MP, Hopkins LG, Thomas M, Greco FA (1998) One-hour paclitaxel plus carboplatin in the treatment of advanced non-small cell lung cancer: results of a multicentre, phase II trial. Eur J Cancer 34:654–658
Helsing M, Thaning L, Sederholm C, Sederholm C, Lamberg K, Martinsson JE, Ek L, Mansson T, Andersson L, Hero U, Anjedani D, Svennson G (1999) Treatment with paclitaxel 1-h infusion and carboplatin of patients with advanced non-small-cell lung cancer: a phase II multicentre trial. Lung Cancer 24:107–113
Huizing MT, Giaccone G, van Warmerdam LJ, Rosing H, Bakker PJ, Vermorken JB, Postmus PE, van Zandwijk N, Koolen MG, Huinink WW, van der Vijgh WJ, Bierhorst FJ, Lai A, Dalesio O, Pinedo HM, Veenhof CH, Beijnen JH (1997) Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer. J Clin Oncol 15:317–329
Jelliffe RW (1973) Creatinine clearance: badside estimate. Ann Intern Med 79:604–605
Johnson DH, Paul DM, Hande KR, Shyr Y, Blanke C, Murphy B, Lewis M, DeVore RF III (1996) Paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a phase II trial. J Clin Oncol 14:2054–2060
Kakolyris S, Kouroussis Ch, Souglakos J, Mavroudis D, Agelaki S, Kalbakis K, Androulakis N, Vardakis N, Vamvakas L, Georgoulias V (2001) A phase I clinical trial of topotecan given every 2 weeks in patients with refractory solid tumors. Oncology 61:265–270
Kearns CM, Gianni L, Egorin MJ (1995) Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol 22 [3 Suppl 6]:16–23
Kelly K, Pan Z, Murphy J, Huffman DH, Bunn PA Jr (1997) A phase I trial of paclitaxel plus carboplatin in untreated patients with advanced non-small cell lung cancer. Clin Cancer Res 3:1117–1123
Kelly K, Crowley J, Bunn PA, Presant CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR, Moore DF, Israel VK, Livingston RB, Gandara DR (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 19:3210–3218
Langer CJ, Leighton JC, Comis RL, O'Dwyer PJ, McAleer CA, Bonjo CA, Engstrom PF, Litwin S, Ozols RF (1995) Paclitaxel and carboplatin in combination in the treatment of advanced non-small-cell lung cancer: a phase II toxicity, response, and survival analysis. J Clin Oncol 13:1860–1870
Roychowdhury DF, Desai P, Zhu YW (1997) Paclitaxel (3-hour infusion) followed by carboplatin (24 hours after paclitaxel): a phase II study in advanced non-small cell lung cancer. Semin Oncol 24 [4 Suppl 12]:37–40
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Shyr Y, Choy H, Cmelak P, Mohr P, Johnson DH (1998) Pattern of practice survey non-small cell lung cancer (NSCLC) in US. Proc Am Soc Clin Oncol 17:463a
Tamura T, Sasaki Y, Nishiwaki Y, Saijo N (1995) Phase I study of paclitaxel by three-hour infusion: hypotension just after infusion is one of the major dose-limiting toxicities. Jpn J Cancer Res 86:1203–1209
WHO (1979) Handbook for reporting results of cancer treatment (offset publication no. 48). World Health Organization, Geneva
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ichiki, M., Gohara, R., Fujiki, R. et al. Phase I and pharmacokinetic study of carboplatin and paclitaxel with a biweekly schedule in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 52, 67–72 (2003). https://doi.org/10.1007/s00280-003-0627-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0627-7