Abstract
Purpose
VX-710 (biricodar, Incel) restores drug sensitivity to cells expressing P-glycoprotein (P-gp) and multidrug resistance-associated protein (MRP1). MRP1 is expressed in a high proportion of prostate tumors while P-gp expression is variable. Since mitoxantrone (M) and prednisone (P) are substrates for MDR transporters, we initiated a study to evaluate the safety, pharmacokinetics, and efficacy of VX-710 plus M/P in patients with hormone-refractory prostate cancer (HRPC).
Patients and methods
Eligible patients had progressive HRPC (defined as new lesions, new disease-related pain, or 50% increase in PSA within 6 weeks of entry), testosterone <30 ng/ml, no prior chemotherapy, ECOG performance status of 0–3, and adequate organ function. Patients received VX-710 (120 mg/m2 per h) as a 72-h continuous intravenous infusion with intravenous bolus mitoxantrone (12 mg/m2) administered 4 h after VX-710 was started and prednisone (5 mg twice daily) administered throughout the study treatment. Endpoints included serum PSA response, PSA response duration, time to PSA progression, pain reduction, and quality of life measures.
Results
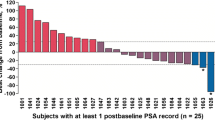
Enrolled in the study were 40 patients and 184 courses of VX-710 plus M/P were administered. Intensive pharmacokinetics, which were performed on six patients who received one cycle of M/P alone, followed by VX-710 plus M/P for all other cycles, showed that VX-710 did not alter mitoxantrone clearance. VX-710 blood concentration at the time of mitoxantrone administration averaged 4.52 μg/ml. VX-710 plus M/P was well tolerated. Transient nausea/vomiting and mild neutropenia were the principal treatment toxicities. Five patients experienced an uncomplicated febrile neutropenic episode (12%), three had severe nausea/vomiting, and two experienced transient moderate to severe ataxia. Of the 40 patients, 12 (30%, 95% confidence interval 16–44%) had a reduction in PSA of ≥50% and 9 of the 12 patients (23% overall, 95% CI 10–35%) achieved a reduction in PSA of ≥80% that was sustained for the duration of treatment with M/P plus VX-710. The median time to PSA progression was 41 weeks (95% CI 34–68 weeks). Of the 40 patients, 15 completed treatment with stable disease and 13 had progressive disease with increasing serum PSA during study treatment. Median survival was 48 weeks for the intent-to-treat population of 40 patients.
Conclusions
The addition of VX-710 to M/P therapy did not appear to increase the proportion of patients with significant serum PSA reductions compared to M/P alone. However, the duration of PSA response observed for the 12 PSA responders suggests that MDR inhibition may benefit some patients with HRPC. In addition to MRP1 or P-gp expression, other mechanisms of drug resistance are probably associated with the relative insensitivity of HRPC to cytotoxic therapy.


Similar content being viewed by others
References
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361
Batra S, Karlsson R, Witt L (1996) Potentiation by estramustine of the cytotoxic effect of vinblastine and doxorubicin in prostatic tumor cells. Int J Cancer 68:644
Bhangal G, Halford S, Wang J, Roylance R, Shah R, Waxman J (2000) Expression of the multidrug resistance gene in human prostate cancer. Urol Oncol 5:118
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92:1295
Bradshaw DM, Arceci RJ (1998) Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. J Clin Oncol 16:3674
Bramwell VH, Morris D, Ernst DS, Hings I, Blackstein M, Venner PM, Ette EI, Harding MW, Waxman A, Demetri GD (2002) Safety and efficacy of the multidrug-resistance inhibitor biricodar (VX-710) with concurrent doxorubicin in patients with anthracycline-resistant advanced soft tissue sarcoma. Clin Cancer Res 8:383–393
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 17:3461
Chaudhary PM, Roninson IB (1991) Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 66:85
Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG (1994) Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res 54:5902
Culine S, Droz JP (2000) Chemotherapy in advanced androgen-independent prostate cancer 1990–1999: a decade of progress? Ann Oncol 11:1523
Drach D, Zhao S, Drach J, Mahadevia R, Gattringer C, Huber H, Andreeff M (1992) Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype. Blood 80:2729
Ehninger G, Schuler U, Proksch B, Zeller KP, Blanz J (1990) Pharmacokinetics and metabolism of mitoxantrone. A review. Clin Pharmacokinet 18:365
Ferry DR, Traunecker H, Kerr DJ (1996) Clinical trials of P-glycoprotein reversal in solid tumours. Eur J Cancer 32A: 1070
Flens MJ, Zaman GJ, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, van der Groep P, de Haas M, Meijer CJ, Scheper RJ (1996) Tissue distribution of the multidrug resistance protein. Am J Pathol 148:1237
Germann UA, Ford PJ, Shlyakhter D, Mason VS, Harding MW (1997) Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing the multidrug resistance-associated protein MRP. Anticancer Drugs 8:141
Germann UA, Shlyakhter D, Mason VS, Zelle RE, Duffy JP, Galullo V, Armistead DM, Saunders JO, Boger J, Harding MW (1997) Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs 8:125
Hipfner DR, Deeley RG, Cole SP (1999) Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta 1461:359
Hudes GR, Nathan F, Khater C, Haas N, Cornfield M, Giantonio B, Greenberg R, Gomella L, Litwin S, Ross E, Roethke S, McAleer C (1997) Phase II trial of 96-hour paclitaxel plus oral estramustine phosphate in metastatic hormone-refractory prostate cancer. J Clin Oncol 15:3156
Izbicka E, Dalton WS, Troyer D, Von Hoff DD (1998) Expression of two multidrug resistance genes in human prostatic carcinomas. J Natl Cancer Inst 90:166
Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V, Trump D, Winer EP, Vogelzang NJ (1999) Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol 17:2506
Kawai K, Sakurai M, Sakai T, Misaki M, Kusano I, Shiraishi T, Yatani R (2000) Demonstration of MDR1 P-glycoprotein isoform expression in benign and malignant human prostate cells by isoform-specific monoclonal antibodies. Cancer Lett 150:147
Kelly WK, Curley T, Slovin S, Heller G, McCaffrey J, Bajorin D, Ciolino A, Regan K, Schwartz M, Kantoff P, George D, Oh W, Smith M, Kaufman D, Small EJ, Schwartz L, Larson S, Tong W, Scher H (2001) Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol 19:44
Lum BL, Gosland MP (1995) MDR expression in normal tissues. Pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematol Oncol Clin North Am 9:319
Oh WK (2000) Chemotherapy for patients with advanced prostate carcinoma: a new option for therapy. Cancer 88:3015
Oh WK, Kantoff PW (1998) Management of hormone refractory prostate cancer: current standards and future prospects. J Urol 160:1220
Oh WK, Kantoff PW (1999) Treatment of locally advanced prostate cancer: is chemotherapy the next step? J Clin Oncol 17:3664
Peck RA, Hewett J, Harding MW, Wang YM, Chaturvedi PR, Bhatnagar A, Ziessman H, Atkins F, Hawkins MJ (2001) Phase I and pharmacokinetic study of the novel MDR1 and MRP1 inhibitor biricodar administered alone and in combination with doxorubicin. J Clin Oncol 19:3130
Petrylak DP, Macarthur RB, O'Connor J, Shelton G, Judge T, Balog J, Pfaff C, Bagiella E, Heitjan D, Fine R, Zuech N, Sawczuk I, Benson M, Olsson CA (1999) Phase I trial of docetaxel with estramustine in androgen-independent prostate cancer. J Clin Oncol 17:958
Rowinsky EK, Smith L, Wang YM, Chaturvedi P, Villalona M, Campbell E, Aylesworth C, Eckhardt SG, Hammond L, Kraynak M, Drengler R, Stephenson J Jr, Harding MW, Von Hoff DD (1998) Phase I and pharmacokinetic study of paclitaxel in combination with biricodar, a novel agent that reverses multidrug resistance conferred by overexpression of both MDR1 and MRP. J Clin Oncol 16:2964
Schummer B, Siegsmund M, Steidler A, Toktomambetova L, Kohrmann KU, Alken P (1999) Expression of the gene for the multidrug resistance-associated protein in human prostate tissue. Urol Res 27:164
Siegsmund MJ, Cardarelli C, Aksentijevich I, Sugimoto Y, Pastan I, Gottesman MM (1994) Ketoconazole effectively reverses multidrug resistance in highly resistant KB cells. J Urol 151:485
Siegsmund MJ, Kreukler C, Steidler A, Nebe T, Kohrmann KU, Alken P (1997) Multidrug resistance in androgen-independent growing rat prostate carcinoma cells is mediated by P-glycoprotein. Urol Res 25:35
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1
Smith DC, Esper P, Strawderman M, Redman B, Pienta KJ (1999) Phase II trial of oral estramustine, oral etoposide, and intravenous paclitaxel in hormone-refractory prostate cancer. J Clin Oncol 17:1664
Sonneveld P (2000) Multidrug resistance in haematological malignancies. J Intern Med 247:521
Speicher LA, Barone LR, Chapman AE, Hudes GR, Laing N, Smith CD, Tew KD (1994) P-glycoprotein binding and modulation of the multidrug-resistant phenotype by estramustine. J Natl Cancer Inst 86:688
Sullivan GF, Amenta PS, Villanueva JD, Alvarez CJ, Yang JM, Hait WN (1998) The expression of drug resistance gene products during the progression of human prostate cancer. Clin Cancer Res 4:1393
Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, Armitage GR, Wilson JJ, Venner PM, Coppin CM, Murphy KC (1996) Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 14:1756
Toppmeyer D, Seidman AD, Pollak M, Russell C, Tkaczuk K, Verma S, Overmoyer B, Garg V, Ette E, Harding MW, Demetri GD (2002) Safety and efficacy of the multidrug resistance inhibitor Incel (biricodar; VX-710) in combination with paclitaxel for advanced breast cancer refractory to paclitaxel. Clin Cancer Res 8:670–678
Van Brussel JP, Van Steenbrugge GJ, Van Krimpen C, Bogdanowicz JF, Van Der Kwast TH, Schroder FH, Mickisch GH (2001) Expression of multidrug resistance related proteins and proliferative activity is increased in advanced clinical prostate cancer. J Urol 165:130
Wiseman LR, Spencer CM (1997) Mitoxantrone. A review of its pharmacology and clinical efficacy in the management of hormone-resistant advanced prostate cancer. Drugs Aging 10:473
Yanagisawa T, Newman A, Coley H, Renshaw J, Pinkerton CR, Pritchard-Jones K (1999) BIRICODAR (VX-710; Incel): an effective chemosensitizer in neuroblastoma. Br J Cancer 80:1190
Yang CP, Shen HJ, Horwitz SB (1994) Modulation of the function of P-glycoprotein by estramustine. J Natl Cancer Inst 86:723
Author information
Authors and Affiliations
Corresponding author
Additional information
In addition to the authors named above, the following Principal Investigators also participated in this work: Robert Shepard, Greater Baltimore Medical Center, Baltimore, MD; Gurkmal Chatta, Arkansas Cancer Research Center, Little Rock, AR; Paul Unger, Vermont Center for Cancer Medicine, Burlington, VT.
Rights and permissions
About this article
Cite this article
Rago, R.P., Einstein, A., Lush, R. et al. Safety and efficacy of the MDR inhibitor Incel (biricodar, VX-710) in combination with mitoxantrone and prednisone in hormone-refractory prostate cancer. Cancer Chemother Pharmacol 51, 297–305 (2003). https://doi.org/10.1007/s00280-003-0573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0573-4