Heading
Abstract
Purpose. Preclinical data indicate that progestational agents (progesterone, medroxyprogesterone acetate and megestrol acetate) interact with p-glycoprotein (P-gp) and reverse P-gp-associated resistance to vinca alkaloids and other natural products. Based on these data, we performed a phase I study of high-dose oral megestrol acetate and vinblastine to evaluate the safety of this regimen.
Patients and methods. Enrolled in the study were 61 patients with advanced solid tumors, refractory to standard therapy. Cohorts of patients received megestrol acetate according to the following escalation scheme (loading dose/maintenance dose, twice daily for 7 days): 750 mg/250 mg, 750 mg/375 mg, 1000 mg/500 mg, 1500 mg/1000 mg, 3000 mg/2000 mg, 4500 mg/3000 mg, 6000 mg/4000 mg, and 7500 mg/5000 mg. They also received 1.5 mg/m2 per day of vinblastine by continuous infusion for 5 days (days 2 to 6).
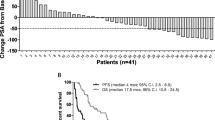
Results. Of the 61 patients, 59 were evaluable for toxicity. A maximum tolerated dose (MTD) was not reached. The regimen was well tolerated. Of the 59 patients, 10 (17%) experienced grade 4 leukopenia. All of these cases were at dose levels 3 to 8. There was an increase in the steady-state concentration (Css) of megestrol acetate with increasing dose up to the sixth dose level. Further increases in the dose produced no change in the megestrol acetate Css. Only 2.4% of megestrol acetate was free in the plasma as compared to 65.6% in RPMI culture medium. Megestrol acetate administration was associated with profound suppression of ACTH and cortisol levels.
Conclusions. The combination of vinblastine and megestrol acetate was well tolerated. An MTD for this combination was not achieved as a result of the saturable absorption of megestrol acetate. Although potentially therapeutic serum concentrations of megestrol acetate were achieved, it is unlikely that MDR was reversed given the high protein-binding of the drug. Profound suppression of the pituitary-adrenal axis was also observed during the administration of megestrol acetate.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Matin, K., Egorin, M.J., Ballesteros, M.F. et al. Phase I and pharmacokinetic study of vinblastine and high-dose megestrol acetate. Cancer Chemother Pharmacol 50, 179–185 (2002). https://doi.org/10.1007/s00280-002-0484-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00280-002-0484-9