Abstract
Multiple myeloma (MM) is the main indication for autologous stem cell transplantation (ASCT). Novel supportive therapies (e.g., granulocyte colony-stimulating factor) have significantly improved post-ASCT-related mortality; however, data on biosimilar pegfilgrastim-bmez (BIO/PEG) in this setting is lacking. This prospective cohort study compared Italian patients with MM who received BIO/PEG post-ASCT with data collected retrospectively from historical control groups from the same center who received either filgrastim-sndz (BIO/G-CSF) or pegfilgrastim (PEG; originator). The primary endpoint was time to neutrophil engraftment (three consecutive days with an absolute neutrophil count ≥ 0.5 × 109/L). Secondary endpoints included incidence and duration of febrile neutropenia (FN). Of the 231 patients included, 73 were treated with PEG, 102 with BIO/G-CSF, and 56 with BIO/PEG. Median age was 60 years and 57.1% were male. Neutrophil engraftment was reached after a median of 10 days in the BIO/PEG and PEG groups and 11 days in the BIO/G-CSF group. Among patients who achieved neutrophil engraftment earlier than this (i.e., day 9), 58% (29/50) were on PEG; of those who achieved it later (i.e., day 11), 80.8% (59/73) were on BIO/G-CSF. FN incidence was higher with BIO/G-CSF (61.4%) versus PEG (52.1%) or BIO/PEG (37.5%) (p = 0.02 among groups). Patients on BIO/PEG had less frequent grade 2–3 diarrhea (5.5%) compared with BIO/G-CSF (22.5%) or PEG (21.9%); grade 2–3 mucositis was most frequent in the BIO/G-CSF group. In conclusion, pegfilgrastim and its biosimilar displayed an advantageous efficacy and safety profile compared with biosimilar filgrastim in patients with MM post-ASCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is the main indication for autologous stem cell transplantation (ASCT) worldwide [1, 2], and high-dose melphalan (HDM) (200 mg/m2) remains the standard conditioning regimen before transplantation [3,4,5,6].
Post-ASCT-related mortality has improved significantly, particularly through new and improved supportive therapies [1, 2, 7]. Use of granulocyte colony-stimulating factors (G-CSF) post-ASCT has been associated with faster neutrophil engraftment in several prospective randomized trials [8, 9], with lower incidences of infection, reduced treatment with broad-spectrum antibiotics, days of hospitalization and treatment costs, and better clinical outcomes [10,11,12,13]. Short-acting filgrastim and long-acting pegfilgrastim, among other biosimilars, are approved treatment options for reducing the duration of neutropenia in patients undergoing myeloablative therapy preceding ASCT, who are at an increased risk of prolonged severe neutropenia [14, 15].
Biosimilars are biologic products that are highly similar to an approved originator product with only minor differences in clinically inactive components and no clinically meaningful differences in efficacy, safety, and purity [16]. Since biosimilars are supported by limited clinical data at the time of approval, data must be extrapolated to support their use in additional indications of the originator product. The first biosimilar ever approved was filgrastim-sndz (Sandoz Inc.), a short-acting G-CSF (BIO/G-CSF) that was approved in 2015 [17]. In 2019, the FDA approved pegfilgrastim-bmez (BIO/PEG; Ziextenzo®, Sandoz Inc.), as a biosimilar of the originator product pegfilgrastim (PEG; Neulasta®, Amgen). Biosimilars such as BIO/G-CSF and BIO/PEG are costly, limiting their accessibility for many patients. The increased need for cost-effective hematopoietic growth factors has recently led to the rapid approval of additional biosimilars [18, 19].
Currently, there are no data describing the use of BIO/PEG post-ASCT in patients with MM. This comparative effectiveness study describes the use of BIO/PEG in patients with MM receiving HDM and undergoing ASCT to assess the relative benefits of BIO/PEG in comparison with historic controls (i.e., BIO/G-CSF and PEG).
Methods
Patients
This prospective cohort study compared patients receiving BIO/PEG with two groups of historical controls (whose data were collected retrospectively), all of whom were referred to the Stem Cell Transplantation Unit of the Grande Ospedale Metropolitano “Bianchi-Melacrino-Morelli” (GOM-BMM) in Reggio Calabria, Italy, for peripheral blood stem cell collection and ASCT. The study was approved by the local institutional review board and conducted according to the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
The study included transplantation-eligible patients who were aged 18–70 years, and had de novo MM, who achieved a favorable response after induction therapy (patient response—complete response (CR) or very good partial response (VGPR) and partial response (PR)—was defined according to the International Myeloma Working Group criteria [20, 21]), were stage 1–3 according to the International Staging System, and who had a World Health Organization performance status of 0–2. Patients were excluded if they met any of the following criteria: non-secretory MM; Waldenstrom macroglobulinemia or immunoglobulin M MM; New York Heart Association class II–IV heart failure; abnormal pulmonary function findings; systematic amyloid light-chain amyloidosis; or a history of active malignancy during the past 5 years (excluding basal cell carcinoma or stage 0 cervical cancer). Patients with an absolute neutrophil count (ANC) of ≤ 1.0 × 109/L, a platelet count of ≤ 75 × 109/L, a creatinine clearance of ≤ 15 mL/min, and those with disease refractory to induction chemotherapy were also excluded.
Treatment
Patients received a bortezomib-based induction therapy; high-dose cyclophosphamide (2–4 g/m2) and G-CSF were administered to mobilize peripheral blood stem cells. The minimum target dose of CD34+ cells required to safely support high-dose chemotherapy was 2 × 106/kg. Post-ASCT, patients received a single, 6-mg subcutaneous injection of BIO/PEG, 24 h after stem cell infusion. These patients were compared with two historical control groups consisting of patients who received either BIO/G-CSF 5 μg/kg/day, from day 5 post-ASCT until neutrophil engraftment, or PEG 6 mg, 24 h after ASCT as a single dose. All patients received oral prophylaxis with levofloxacin 500 mg/day from day 0 until neutrophil engraftment and acyclovir 800 mg twice daily from day 3 until approximately day 90 post-ASCT. Pneumocystis jirovecii pneumonia prophylaxis was administered with trimethoprim/sulfamethoxazole (1 double strength tablet; 2–3 times weekly), initiated post-hematologic recovery for 3 months. Cryotherapy (ice chips) was utilized to prevent HDM-induced oral mucositis; the patients placed ice chips in their mouths approximately 30 min before and 6 h after HDM. Red blood cell (RBC) and platelet transfusions were administered to maintain hemoglobin levels of ≥ 8 mg/dL and platelet counts of ≥ 10 × 109/L or in patients with symptomatic anemia/minimal mucocutaneous hemorrhagic syndrome. Intravenous hydration and electrolyte support was also provided. Where febrile neutropenia (FN) occurred following a long period of neutropenia (ANC < 0.5 × 109/L or ANC of 1 × 109/L with a predicted decline to < 0.5 × 109/L over the subsequent 48 h) blood and catheter-drawn cultures were ordered, and intravenous ceftriaxone was promptly started.
Endpoints
The primary endpoint of this study was time to neutrophil engraftment, defined as three consecutive days where the patient had an ANC of ≥ 0.5 × 109/L.
Secondary endpoints included the incidence and duration of FN, and the incidence of mucositis, diarrhea, and platelet engraftment (platelet count ≥ 20 × 109/L, not requiring a platelet transfusion in the preceding 7 days). Complete blood counts were conducted using blood samples collected before chemotherapy and daily during the aplastic phase until hospital discharge. FN was defined as a temperature of ≥ 38.2°C on at least two consecutive occasions, or a persistent temperature of ≥ 38.0°C for at least 1 h, accompanied by an ANC of < 0.5 × 109/L in the absence of any documented noninfectious cause (e.g., transfusion reaction or administration of cytotoxic drugs).
The safety endpoint of the study was the incidence of study drug-related adverse events.
Statistical analysis
Descriptive statistics were used to present data, including median, interquartile range, and percentage values. Between-group comparisons were performed using the Kruskal–Wallis or chi-square tests, as appropriate. Comparative analyses were used to identify covariates to be included in multiple models as associated with growth factor treatment (BIO/PEG vs PEG; BIO/PEG vs BIO/G-CSF) or FN (yes/no). Accounting for different time frames of drug administration (BIO/PEG was introduced in the last 3 years) and changing treatment guidelines, basal CD34+ infusion was dichotomized based on a clinical threshold over the whole period (< 4 and ≥ 4 × 106/kg).
To assess the relationship between time to neutrophil engraftment and other patient variables, univariate Kaplan–Meier analyses were conducted. As the proportional hazard assumption was violated, the restricted mean survival time (RMST) was adopted to estimate the treatment effect. RMST, defined as the area under the survival function curve up to a specific time (t*), shows the mean survival time or, in our case, the mean time in which there was no neutrophil engraftment. The difference in RMST (∆RMST) can be described as change (gain or loss) in event-free survival time between treatment groups during this specific timeframe. There were no censored observations in this study, so RMST corresponded to the mean survival time and t* corresponded to the total timeframe. To account for differences in follow-up duration between the three treatment groups, a sensitivity analysis was performed over a predefined period (13 days).
The relationship between FN and other patient variables was evaluated by univariate logistic regression analysis; identified covariates were used for multiple logistic regression analysis. For the logistic models, data were expressed as odds ratio (OR), 95% confidence intervals (CI), and p-values. All analyses were adjusted by patient sex and age, irrespective of the association with the outcome (significant/not significant).
Statistical analyses were performed with the survRM2 and temporal packages in the software R, version 3.6.3.
Results
Study population
From January 2021 to June 2022, 56 consecutive patients with MM underwent HDM followed by ASCT and administration of BIO/PEG. Two historical control groups consisted of 102 patients who underwent transplantation in 2016–2018 and received BIO/G-CSF, and 73 patients who underwent transplantation between 2019 and 2020 and received PEG, both after stem cell infusion.
Table 1 summarizes patient characteristics at the time of ASCT. Patients in all three groups received the same HDM schedule and were adequately matched to historic controls with respect to baseline demographic and clinical characteristics. The proportion of patients with a CR or VGPR and PR was 98.2% (n = 55) and 1.8% (n = 1) in the BIO/PEG group, 89.2% (n = 91) and 10.8% (n = 11) in the BIO/G-CSF group, and 95.9% (n = 70) and 4.1% (n = 3) in the PEG group, respectively.
All patients were in first line of treatment and received 4–6 cycles of a bortezomib induction therapy before mobilization.
Transfusions
The proportion of patients who underwent RBC or platelet transfusion did not differ between the three treatment groups (Table 2), whereas the median number of platelet transfusions among platelet-transfused patients was twofold higher in PEG-treated patients than in the remaining two groups. There was no difference in the number of RBC transfusions among RBC-transfused patients across the three groups (Table 2).
Time to neutrophil engraftment
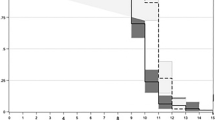
Figure 1 shows the absolute number of patients achieving neutrophil engraftment (ANC ≥ 0.5 × 109/L) across all three treatment groups over time. In the PEG, BIO/PEG, and BIO/G-CSF groups, the highest proportion of patients achieved neutrophil engraftment at days 9, 10, and 11, respectively. The median time to neutrophil engraftment was 10 days in the BIO/PEG and PEG groups, and 11 days in the BIO/G-CSF group. Among patients who achieved neutrophil engraftment early relative to the median (i.e., day 9; n = 50), 29 patients (58.0%) were treated with PEG, 18 (36.0%) with BIO/PEG, and three (6.0%) with BIO/G-CSF (Fig. 1).
Absolute number of patients reaching neutrophil engraftment (absolute neutrophil count ≥ 0.5 × 109/L) across all three treatment groups over time. ASCT, autologous stem cell transplantation; BIO/G-CSF, biosimilar granulocyte colony-stimulating factor (filgrastim-sndz); BIO/PEG, biosimilar pegfilgrastim (pegfilgrastim-bmez); n, number; PEG, pegfilgrastim
Among those who achieved neutrophil engraftment at day 10 (n = 69), 28 patients (40.5%) were treated with PEG, 25 patients (36.2%) with BIO/PEG, and 16 patients (23.1%) with BIO/G-CSF. Among those who achieved neutrophil engraftment relatively late (i.e., day 11; n = 73), 59 patients (80.8%) were treated with BIO/G-CSF, nine (12.3%) with PEG, and five (6.8%) with BIO/PEG.
The time to reach neutrophil engraftment was further investigated using a Kaplan–Meier analysis (Fig. 2). Cumulative survival-free time of neutrophil engraftment did not differ between patients on BIO/PEG and PEG (log rank test, p = 0.33) but was significantly shorter in these groups than in patients treated with BIO/G-CSF (log rank test, p < 0.001); i.e., time to neutrophil engraftment was shorter in patients who were treated with BIO/PEG and PEG.
A RMST analysis further confirmed that neutrophil engraftment occurred, on average, 1 day later (p < 0.001) in patients treated with BIO/G-CSF than in those treated with BIO/PEG (∆RMST0.99 days; 95% CI 0.66–1.33) and PEG (1.11 days; 95% CI 0.63–1.6); no differences were found between the BIO/PEG and PEG groups (p = 0.68; Table 3). Age- and sex-adjusted analyses also confirmed these results.
To account for differences in time duration between the three treatment groups, a landmark analysis was performed (Supplementary Table S1; Online Resource 1). This sensitivity analysis was carried out over a 13-day period and showed similar results to those reported in the RMST analysis.
Febrile neutropenia
FN occurred in 52.4% of patients (Table 2). The incidence of FN was higher in patients on BIO/G-CSF (61.4%; 95% CI 52–71%) than those on PEG (52.1%; 95% CI 40–64%) and BIO/PEG (37.5%; 95% CI 25–51%) (p = 0.02 among groups). A direct comparison of FN incidence showed that this complication occurred in significantly fewer patients in the BIO/PEG group than in the BIO/G-CSF group (37.5% vs 61.4%, p < 0.01). The incidence of FN did not differ significantly between patients in the PEG and BIO/PEG groups (p = 0.14) or between those in the PEG and BIO/G-CSF groups (p = 0.28).
Among patients with FN, there was no difference in the number of days with fever between the three treatment groups (Table 2). The incidence of fever of unknown origin (FUO) was higher in patients in the PEG group (94.7%) than in the BIO/G-CSF (83.6%) or BIO/PEG (47.6%) groups (Table 2). The incidence of FN did not differ by patient age, sex, or amount of stem cells infused (Table 4).
As the incidence of FN did not significantly differ between the PEG and BIO/PEG groups, these two groups of patients were combined and compared with the BIO/G-CSF group. In both the univariate and multiple logistic regression analyses, the odds of FN occurring were approximately twofold higher in the BIO/G-CSF group than in the BIO/PEG or PEG groups (Table 5).
Mucositis and diarrhea
Significant differences were found in the incidence of mucositis and diarrhea across the three treatment groups (Table 2). Specifically, patients in the BIO/PEG group had a lower incidence of grade 2–3 diarrhea (5.5%) than those in the other two treatment groups (BIO/G-CSF: 22.5%; PEG: 21.9%). The incidence of grade 2–3 mucositis was not significantly different between the BIO/PEG and PEG groups (7.3% vs 9.6%), with a higher incidence in the BIO/G-CSF group (34.3%). No deaths occurred in this study.
Safety
Mild bone pain was observed in 49.3% (n = 36/73) of patients taking BIO/G-CSF and in approximately 10% of patients taking PEG (n = 11/102) and BIO/PEG (n = 6/56). Bone pain occurred largely on days of neutrophil engraftment. In most patients, pain symptoms were controlled by the administration of paracetamol. No cardiac, neurological, renal, or pulmonary complications were reported, and no patients died in the first 100 days post-transplantation.
Discussion
In this study, the use of BIO/PEG in patients with MM undergoing ASCT resulted in achievement of neutrophil engraftment (ANC ≥ 0.5 × 109/L) in a median of 10 days, similar to that observed in the historical PEG group. Among patients achieving engraftment earlier, 58.0% were treated with PEG. Furthermore, the use of pegylated G-CSF was associated with a statistically significant faster neutrophil engraftment, as reported in other studies [22, 23].
Current data describing the use of BIO/PEG in patients with MM post-ASCT are scarce. A 2021 study by Wang and colleagues reported that among patients with MM who underwent ASCT, the mean time to neutrophil engraftment was 8.72 days in patients treated with BIO/PEG versus 9.87 days in those who received BIO/G-CSF [24]. Similarly, a comparative study by Vanstraelen and colleagues reported a median time to neutrophil engraftment of 8 and 9 days in patients treated with BIO/PEG and BIO/G-CSF, respectively [25]. The decreased time to neutrophil engraftment in the reported studies may have resulted from variability in a range of factors known to affect engraftment kinetics, including population age, treatment timing, conditioning regimen, stage of disease, and prior melphalan exposure [26, 27]. Combined with our results, albeit in a limited dataset, these data suggest that BIO/PEG has improved efficacy compared with BIO/G-CSF in patients with MM who are undergoing ASCT. In the current study, the cumulative survival-free time until neutrophil engraftment did not differ between patients on BIO/PEG and PEG but was significantly shorter in these groups than among patients treated with BIO/G-CSF. Of note, a pivotal single-dose, three-period crossover study by Bellonand colleagues, which compared BIO/PEG and PEG in healthy adults, found similar pharmacokinetic and pharmacodynamic profiles between the originator and its biosimilar [28]. The equivalent pharmacodynamic profiles observed were indicative of equivalent clinical efficacy, as shown in a confirmatory study of both agents in patients with breast cancer [29]. In the current study, RMST analysis of time to neutrophil engraftment found no significant difference between patients on BIO/PEG and PEG, which is in line with previous studies.
The lowest incidence of FN was in the BIO/PEG group, intermediate in PEG and highest in the BIO/G-CSF, which is also consistent with previously reported clinical data [25, 30, 31]. Although slight differences in patient populations, treatment regimens, and timing/duration of drug administration may result in small variabilities in FN incidence, our data likely reflect the real-world outcomes of patients receiving cytotoxic chemotherapy [32]. The incidence of FN in this study did not differ significantly between patients in the PEG and BIO/PEG groups. Whilst comparative data on these two biosimilars are currently lacking, our observations may in part be supported by the demonstrated pharmacodynamic and pharmacokinetic similarity of the two agents [28].
The incidence of FUO was higher in patients on PEG (94.7%) compared with patients on BIO/G-CSF (83.6%) or BIO/PEG (47.6%). These results differed from those reported by Castagna and colleagues, whereby the FUO incidence was higher with BIO/G-CSF (62%) than with PEG (56%) [33]. This discrepancy between our study and that of Castagna and colleagues may be explained by the latter study including patients with a range of hematologic malignancies and solid tumors, with varying disease stages and differing prior treatments. Further studies into the variables effecting incidence of FUO are warranted.
Patients treated with BIO/PEG had a lower incidence of grade 2–3 diarrhea compared with those on the other two treatments, which is in line with previous observations [29]. The incidence of mucositis was significantly higher in patients treated with BIO/G-CSF than in those treated with BIO/PEG or PEG.
In vivo studies have found that upon administration, recombinant human G-CSF causes an initial transient decrease in peripheral neutrophils [34]. Additionally, peak neutrophil counts typically occur approximately 12 h after BIO/G-CSF administration, after which there is a decline over 2–3 days compared with PEG in which neutrophil counts decline slowly over 1–2 weeks [34]. Further investigation of this hypothesis in prospective, randomized studies is warranted. Finally, bone pain was most commonly observed in patients treated with BIO/G-CSF, consistent with previous reports [35].
Filgrastim is usually administered as a series of daily injections after a chemotherapy cycle, whereas pegfilgrastim (both the original formulation and the biosimilar) is injected once-off, as a single dose, with each chemotherapy cycle. With the convenience of a once-off administration per cycle, BIO/PEG could become the future standard-of-care in an outpatient program for HDM and supportive care after ASCT in patients with MM. This reduced frequency of dosing could result in significant time savings for staff when compared to scheduled daily injections [36,37,38]. In our study, the pegylated formulation of G-CSF was more effective than BIO/G-CSF as prophylaxis for FN. However, in clinical practice, its usage has been limited because of its higher cost, as the benefit of PEG is unclear compared to its associated cost. Most studies showed that PEG is cost-effective compared with filgrastim as primary and secondary prophylaxis for chemotherapy-induced FN inpatients with lymphoma [39]. Our study did not aim to perform a cost benefit analysis, but it is plausible that there may be an economic advantage in using the pegylated biosimilar formulation in the setting of patients undergoing ASCT [40]. Our findings could assist clinicians and healthcare decision-makers to make informed decisions regarding resource allocation for the management of chemotherapy-induced FN in settings similar to those studied.
Our study has some limitations, including the use of historic control arms. Use of such controls is likely to produce unfair comparisons and confounding variables as we cannot control for differences in patient characteristics between the intervention and control groups. Although control groups were carefully selected to maximize comparability, these results should be extrapolated with caution as the registry data does not account for specific confounders and represents a limited sample size, potentially impacting the efficacy outcomes.
In conclusion, PEG and its biosimilar (BIO/PEG) are advantageous, demonstrating improved efficacy and a superior safety profile compared with BIO/G-CSF in patients with MM who were conditioned with HDM prior to ASCT. Indeed, our work showed a shorter time to reach neutrophil engraftment, a lower incidence of FN, a lower incidence of grade 2–3 diarrhea, and a lower incidence of grade 2–3 mucositis in patients treated with PEG and BIO/PEG. Additional clinical trials, rather than observational studies, would facilitate further acceptance and use of such biosimilars in patients with MM receiving HDM and undergoing ASCT in routine clinical practice.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
22 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00277-023-05323-1
References
Auletta JJ, Kou J, Chen M, Shaw BE (2021) Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx. Accessed 12 December 2022
Passweg JR, Baldomero H, Chabannon C, Corbacioglu S, de la Cámara R, Dolstra H et al (2022) Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant 57:742–752. https://doi.org/10.1038/s41409-022-01604-x
Blanes M, Lorenzo JI, Ribas P, Jiménez A, González JD, Cejalvo MJ et al (2019) Intravenous busulfan plus melphalan versus melphalan alone as conditioning regimen for patients with multiple myeloma. Ann Hematol 98:2013–2015. https://doi.org/10.1007/s00277-019-03663-5
Martino M, Tripepi G, Messina G, Vincelli ID, Console G, Recchia AG et al (2016) A phase II, single-arm, prospective study of bendamustine plus melphalan conditioning for second autologous stem cell transplantation in de novo multiple myeloma patients through a tandem transplant strategy. Bone Marrow Transplant 51:1197–1203. https://doi.org/10.1038/bmt.2016.94
Musso M, Messina G, Marcacci G, Crescimanno A, Console G, Donnarumma D et al (2015) High-dose melphalan plus thiotepa as conditioning regimen before second autologous stem cell transplantation for "de novo" multiple myeloma patients: a phase II study. Biol Blood Marrow Transplant 21:1932–1938. https://doi.org/10.1016/j.bbmt.2015.06.011
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D et al (2010) Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood 115:32–37. https://doi.org/10.1182/blood-2009-06-229658
Bhatt VR, Loberiza FR Jr, Jing H, Bociek RG, Bierman PJ, Maness LJ et al (2015) Mortality patterns among recipients of autologous hematopoietic stem cell transplantation for lymphoma and myeloma in the past three decades. Clin Lymphoma Myeloma Leuk 15:409–15.e1. https://doi.org/10.1016/j.clml.2015.02.024
Lee SM, Radford JA, Dobson L, Huq T, Ryder WD, Pettengell R et al (1998) Recombinant human granulocyte colony-stimulating factor (filgrastim) following high-dose chemotherapy and peripheral blood progenitor cell rescue in high-grade non-Hodgkin's lymphoma: clinical benefits at no extra cost. Br J Cancer 77:1294–1299. https://doi.org/10.1038/bjc.1998.216
Linch DC, Milligan DW, Winfield DA, Kelsey SM, Johnson SA, Littlewood TJ et al (1997) G-CSF after peripheral blood stem cell transplantation in lymphoma patients significantly accelerated neutrophil recovery and shortened time in hospital: results of a randomized BNLI trial. Br J Haematol 99:933–938. https://doi.org/10.1046/j.1365-2141.1997.4703274.x
Lalami Y, Klastersky J (2017) Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: an overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol 120:163–179. https://doi.org/10.1016/j.critrevonc.2017.11.005
Lyman GH, Kuderer NM (2003) Epidemiology of febrile neutropenia. Support Cancer Ther 1:23–35. https://doi.org/10.3816/SCT.2003.n.002
Singh AD, Parmar S, Patel K, Shah S, Shore T, Gergis U et al (2018) Granulocyte colony-stimulating factor use after autologous peripheral blood stem cell transplantation: comparison of two practices. Biol Blood Marrow Transplant 24:288–293. https://doi.org/10.1016/j.bbmt.2017.10.026
Trivedi M, Martinez S, Corringham S, Medley K, Ball ED (2009) Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant 43:895–908. https://doi.org/10.1038/bmt.2009.75
U.S. Food and Drug Administration (2015) ZARXIO®(filgrastim-sndz) injection, for subcutaneous or intravenous use: highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125553s007lbl.pdf. Accessed 14 February 2023
U.S. Food and Drug Administration (2019) ZIEXTENZO™ (pegfilgrastim-bmez) injection, for subcutaneous use: highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761045lbl.pdf. Accessed 14 February 2023
U.S. Food and Drug Administration (2017) Biosimilar and interchangeable products. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products. Accessed 16 December 2019
Awad M, Singh P, Hilas O (2017) Zarxio (Filgrastim-sndz): the first biosimilar approved by the FDA. P & T 42:19–23
Lyman GH, Zon R, Harvey RD, Schilsky RL (2018) Rationale, opportunities, and reality of biosimilar medications. N Engl J Med 378:2036–2044. https://doi.org/10.1056/NEJMhle1800125
U.S. Food and Drug Administration (2010) Implementation of the biologics price competition and innovation act of 2009. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/implementation-biologics-price-competition-and-innovation-act-2009.
Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473. https://doi.org/10.1038/sj.leu.2404284
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23:3–9. https://doi.org/10.1038/leu.2008.291
Sheth V, Gore A, Jain R, Ghanekar A, Saikia T (2019) Pegfilgrastim: more cost effective and equally efficacious option as compared to filgrastim in autologous stem cell transplant. Indian J Hematol Blood Transfus 35:66–71. https://doi.org/10.1007/s12288-018-0966-5
Wannesson L, Luthi F, Zucca E, Rosselet-Christ A, Baglioni M, Marelli L et al (2011) Pegfilgrastim to accelerate neutrophil engraftment following peripheral blood stem cell transplant and reduce the duration of neutropenia, hospitalization, and use of intravenous antibiotics: a phase II study in multiple myeloma and lymphoma and comparison with filgrastim-treated matched controls. Leuk Lymphoma 52:436–443. https://doi.org/10.3109/10428194.2010.545462
Wang X, Ren J, Liang X, He P (2021) Efficacy and cost of G-CSF derivatives for prophylaxis of febrile neutropenia in lymphoma and multiple myeloma patients underwent autologous hematopoietic stem cell transplantation. Hematology 26:950–955. https://doi.org/10.1080/16078454.2021.2003071
Vanstraelen G, Frère P, Ngirabacu MC, Willems E, Fillet G, Beguin Y (2006) Pegfilgrastim compared with filgrastim after autologous hematopoietic peripheral blood stem cell transplantation. Exp Hematol 34:382–388. https://doi.org/10.1016/j.exphem.2005.11.013
Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL et al (2009) International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia 23:1904–1912. https://doi.org/10.1038/leu.2009.127
Hassan MN, Fauzi HM, Husin A, Mustaffa R, Hassan R, Ibrahim MI et al (2019) Autologous peripheral blood stem cell transplantation among lymphoproliferative disease patients: factors influencing engraftment. Oman Med J 34:34–43. https://doi.org/10.5001/omj.2019.06
Bellon A, Wang J, Skerjanec A, Velinova M, Dickerson D, Sabet A et al (2020) A large multicentre, randomized, double-blind, cross-over study in healthy volunteers to compare pharmacokinetics, pharmacodynamics and safety of a pegfilgrastim biosimilar with its US- and EU-reference biologics. Br J Clin Pharmacol 86:1139–1149. https://doi.org/10.1111/bcp.14226
Blackwell K, Gascon P, Jones CM, Nixon A, Krendyukov A, Nakov R et al (2017) Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28:2272–2277. https://doi.org/10.1093/annonc/mdx303
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL (2011) Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404. https://doi.org/10.1186/1471-2407-11-404
Pfeil AM, Allcott K, Pettengell R, von Minckwitz G, Schwenkglenks M, Szabo Z (2015) Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Sup Care Cancer 23:525–545. https://doi.org/10.1007/s00520-014-2457-z
Kitchen K, Mosier M (2021) Real-world comparison of febrile neutropenia rates with same-day versus next-day administration of pegfilgrastim. J Clin Oncol 39:299. https://doi.org/10.1200/JCO.2020.39.28_suppl.299
Castagna L, Bramanti S, Levis A, Michieli MG, Anastasia A, Mazza R et al (2010) Pegfilgrastim versus filgrastim after high-dose chemotherapy and autologous peripheral blood stem cell support. Ann Oncol 21:1482–1485. https://doi.org/10.1093/annonc/mdp576
Page AV, Liles WC (2020) Immunomodulators. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Elsevier, Inc, pp 627–54.e7
Neumann TA, Foote M (2012) The safety profile of filgrastim and pegfilgrastim. In: Molineux G, Foote M, Arvedson T (eds) Twenty Years of G-CSF. Milestones in Drug Therapy. Springer, Basel, pp 395–408
Martino M, Paviglianiti A, Memoli M, Martinelli G, Cerchione C (2020) Multiple myeloma outpatient transplant program in the era of novel agents: state-of-the-art. Front Oncol 10:592487. https://doi.org/10.3389/fonc.2020.592487
Martino M, Russo L, Martinello T, Gallo GA, Fedele R, Moscato T et al (2015) A home-care, early discharge model after autografting in multiple myeloma: results of a three-arm prospective, non-randomized study. Leuk Lymphoma 56:801–804. https://doi.org/10.3109/10428194.2014.931952
Martino M, Montanari M, Ferrara F, Ciceri F, Scortechini I, Palmieri S et al (2014) Very low rate of readmission after an early discharge outpatient model for autografting in multiple myeloma patients: an Italian multicenter retrospective study. Biol Blood Marrow Transplant 20:1026–1032. https://doi.org/10.1016/j.bbmt.2014.03.027
Gebremariam GT, Fentie AM, Beyene K, Sander B, Gebretekle GB (2022) Cost-effectiveness of pegfilgrastim versus filgrastim for prevention of chemotherapy-induced febrile neutropenia in patients with lymphoma: a systematic review. BMC Health Serv Res 22:1600. https://doi.org/10.1186/s12913-022-08933-z
Wang W, Li E, Campbell K, McBride A, D'Amato S (2021) Economic analysis on adoption of biosimilar granulocyte colony-stimulating factors in patients with nonmyeloid cancer at risk of febrile neutropenia within the oncology care model framework. JCO Oncol Pract 17:e1139–e1e49. https://doi.org/10.1200/OP.20.00994
Acknowledgements
The authors would like to thank Arneak Kooner of Springer Healthcare Communications who provided editorial assistance (writing the abstract, editing the main body of the text, styling for submission) in preparing this manuscript. Medical writing was supported by Sandoz.
Funding
Editorial assistance in the preparation of this article was supported by Sandoz.
Author information
Authors and Affiliations
Contributions
Conceptualization: Massimo Martino, Filippo Antonio Canale, Barbara Loteta, Giuseppe Console, Virginia Naso, Antonella Morabito, Maria Altomonte, Maria Teresa Florenzano, Maria Consuelo Ieracitano, Amelia Cuzzocrea, Anna Ferreri, Tiziana Moscato, Giuseppe Irrera, Caterina Alati, and Marta Pugliese; methodology: Massimo Martino, Gaetana Porto, Maria Pellicano, Ludovica Santoro, Chiara Verduci, Mercedes Gori, Annalisa Pitino, and Giovanni Tripepi; formal analysis and investigation: Giovanni Tripepi, Annalisa Pitino, and Mercedes Gori; writing—original draft: Massimo Martino, Mercedes Gori, Annalisa Pitino, and Giovanni Tripepi; writing—review and editing: Massimo Martino, Mercedes Gori, Annalisa Pitino, and Giovanni Tripepi; supervision: Massimo Martino. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the International Conference on Harmonization Guidelines for Good Clinical Practice and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the local institutional review board.
Consent to participate
All participants provided written informed consent at enrolment.
Consent to publish
All participants provided written informed consent for the publication of their data.
Competing interests
The authors declare no competing interests.
Authorship
All listed authors have made substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data, or the creation of new software used in the work; have drafted the work or revised it critically for important intellectual content; have approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Massimo Martino and Mercedes Gori contributed equally as first authors.
Giovanni Tripepi and Annalisa Pitino contributed equally as last authors.
Supplementary Information
ESM 1:
Table S1 Restricted mean survival time (RMST) estimates and ΔRMST of univariate and multiple models within 13 days
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martino, M., Gori, M., Porto, G. et al. Effectiveness of biosimilar pegfilgrastim in patients with multiple myeloma after high-dose melphalan and autologous stem cell transplantation. Ann Hematol 102, 1915–1925 (2023). https://doi.org/10.1007/s00277-023-05228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05228-z