Abstract
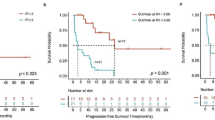
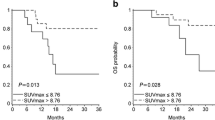
Chimeric antigen receptor (CAR) T-cell therapy provides long-term remissions in patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL). Total metabolic tumor volume (TMTV) assessed by 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) has a confirmed prognostic value in the setting of chemoimmunotherapy, but its predictive role with CAR T-cell therapy is not fully established. Thirty-five patients with R/R LBCL who received CAR T-cells were included in the study. TMTV and maximum standardized uptake value (SUVmax) were measured at baseline and 1-month after CAR T-cell infusion. Best response included 9 (26%) patients in complete metabolic response (CMR) and 16 (46%) in partial metabolic response (PMR). At a median follow-up of 7.6 months, median PFS and OS were 3.4 and 8.2 months, respectively. A high baseline TMTV (≥ 25 cm3) was associated with a lower PFS (median PFS, 2.3 vs. 8.9 months; HR = 3.44 [95% CI 1.18–10.1], p = 0.02). High baseline TMTV also showed a trend towards shorter OS (HR = 6.3 [95% CI 0.83–47.9], p = 0.08). Baseline SUVmax did not have a significant impact on efficacy endpoints. TMTV and SUVmax values showed no association with adverse events. Metabolic tumor burden parameters measured by 18FDG-PET before CAR T-cell infusion can identify LBCL patients who benefit most from this therapy.




Similar content being viewed by others
References
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J et al (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130(16):1800–1808
González-Barca E, Boumendil A, Blaise D, Trněný M, Masszi T, Finel H et al (2020) Outcome in patients with diffuse large B-cell lymphoma who relapse after autologous stem cell transplantation and receive active therapy. A retrospective analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 55(2):393–9
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA et al (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26):2531–2544
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380(1):45–56
Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K et al (2020) Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol 38(27):3095–3106
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y et al (2020) Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol 38(27):3119–3128
Vercellino L, Cottereau AS, Casasnovas O, Tilly H, Feugier P, Chartier L et al (2020) High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood 135(16):1396–1405
Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W et al (2019) Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor t cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant 25(6):1092–1098
Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M et al (2020) Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv 4(22):5607–5615
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN et al (2019) ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25(4):625–638
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32(27):3048–3058
Meignan M, Gallamini A, Haioun C (2009) Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma 50:1257–1260 (United States)
Barrington SF, Qian W, Somer EJ, Franceschetto A, Bagni B, Brun E et al (2010) Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37(10):1824–1833
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42(2):328–54
Klein JP, Moeschberger ML (2003) Survival analysis techniques for censored and truncated data. Springer
Harrell FEJ. (2016) Package ‘rms’ (The Comprehensive R Archive Network)
Dafni U (2011) Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes 4(3):363–71
Cottereau AS, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H et al (2016) Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res 22(15):3801–3809
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A et al (2014) Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 41(11):2017–2022
Guo B, Tan X, Ke Q, Cen H (2019) Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: a meta-analysis. PLoS ONE 14(1):e0210224
Cottereau A-S, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R et al (2018) Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 131(13):1456–1463
Dean EA, Mhaskar RS, Lu H, Mousa MS, Krivenko GS, Lazaryan A et al (2020) High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv 4(14):3268–3276
Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R et al (2020) Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4(21):5414–5424
Sesques P, Ferrant E, Safar V, Wallet F, Tordo J, Dhomps A et al (2020) Commercial anti-CD19 CAR T cell therapy for patients with relapsed/refractory aggressive B cell lymphoma in a European center. Am J Hematol 95(11):1324–1333
Shah NN, Nagle SJ, Torigian DA, Farwell MD, Hwang WT, Frey N et al (2018) Early positron emission tomography/computed tomography as a predictor of response after CTL019 chimeric antigen receptor -T-cell therapy in B-cell non-Hodgkin lymphomas. Cytotherapy 20(12):1415–1418
Hossain NM, Dahiya S, Le R, Abramian AM, Kong KA, Muffly LS et al (2019) Circulating tumor DNA assessment in patients with diffuse large B-cell lymphoma following CAR T-cell therapy. Leuk Lymphoma 60(2):503–506
Sworder B, Kurtz DM, Macaulay C, Frank MJ, Alig S, Garofalo A et al (2019) Circulating DNA for molecular response prediction, characterization of resistance mechanisms and quantification of CAR T-cells during axicabtagene ciloleucel therapy. Blood 134(Supplement_1):550
Frank MJ, Hossain N, Bukhari A, Dean E, Spiegel JY, Claire GK et al (2019) Detectable circulating tumor DNA 28 days after the CD19 CAR T-cell therapy, axicabtagene ciloleucel, is associated with poor outcomes in patients with diffuse large B-cell lymphoma. Blood 134(Supplement_1):884
Acknowledgements
The authors thank the patients and their families for their participation in this study.
Author information
Authors and Affiliations
Contributions
Concept and design were undertaken by PB, GI, and MS. Data analysis and interpretation were performed by GV, MS, PB, and GI. Collection and assembly of data were performed by GI, PB, CC, and EC. All authors contributed to manuscript writing and final approval of the manuscript, and are accountable for all aspects of the work (ensuring questions related to accuracy or integrity of the work are appropriately investigated and resolved).
Corresponding author
Ethics declarations
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Conflict of interest
G.I. declares having received honoraria from Celgene, Gilead, Novartis, and Roche, not related with the present article. G.V. reported receiving honoraria for speaker activities from Merck Sharp & Dohme and advisory role from Astrazeneca. E.C. declares having no conflict of interest. C.C. declares having received honoraria from Takeda and Regeneron, not related with the present article. C. D-L declares having received honoraria from Celgene and Novartis, not related with the present article. A.V.J. received funding from Fondo de Investigaciones Sanitarias and Instituto de Salud Carlos III (FIS PI17/02162); and has engaged in consulting and/or participated as speaker in events organized by Novartis, Roche, Teva, Mylan, Biogen, Merck, and Sanofi. A.P. declares having no conflict of interest. M.J. declares having no conflict of interest. P.A. declares having received honoraria from Celgene, Gilead, Janssen, Abbvie, and Roche, not related with the present article. F.B. declares having received honoraria from Celgene, Gilead, Novartis, Pfizer, and Roche, not related with the present article. P.B. declares having received honoraria from Amgen, Celgene, Gilead, Incyte, Jazz Pharmaceuticals, MSD, Novartis, Pfizer, and Roche, not related with the present article. P.B. received funding from the Carlos III FIS16/01433 Health Institute, Asociación Española contra el Cáncer (Ideas Semilla 2019) and a PERIS 2018–2020 grant from the Generalitat de Catalunya (BDNS357800).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iacoboni, G., Simó, M., Villacampa, G. et al. Prognostic impact of total metabolic tumor volume in large B-cell lymphoma patients receiving CAR T-cell therapy. Ann Hematol 100, 2303–2310 (2021). https://doi.org/10.1007/s00277-021-04560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04560-6