Abstract
High-dose methotrexate (HD-MTX) at 3 g/m2 is one of the strategies for central nervous system (CNS) prophylaxis in the first-line treatment of aggressive lymphomas, especially in diffuse large B cell lymphoma patients with high-risk CNS-International Prognostic Index. The objective of our study was to retrospectively analyze the safety of 2 cycles of systemic HD-MTX administered as an ambulatory regimen. Between January 2013 and December 2016, 103 patients were carefully selected on 6 criteria, including age < 60, albumin > 34, performance status 0 or 1, normal renal and hepatic functions, good understanding of practical medical guidance, and no loss of weight. Strict procedures of HD-MTX infusion were observed including alkalinization, urine pH monitoring, and leucovorin rescue. Renal and hepatic functions were monitored at days 2 and 7. MTX clearance was not monitored. Toxicities and grades of toxicity were collected according to the NCI-CTCAE (version 4.0). Among the 103 selected patients, 92 (89%) patients successfully completed the planned 2 cycles of HD-MTX on an outpatient basis. Eleven patients completed only 1 cycle, 3 because of lymphoma progression and 8 because of toxicity including 3 grade II hepatotoxicity, 2 grade I/II renal toxicity, 1 grade III neutropenia, 1 active herpetic infection, and 1 grade III ileus reflex. Reported adverse events (AE) included 92 (84%) grade I/II and 18 (16%) grade III/IV. Grade III hepatotoxicity, mostly cytolysis, was the most frequent AE observed with 8 (8%) events. Grade III/IV hematologic toxicities concerned 9 patients with 8 grade III/IV neutropenia and 1 thrombocytopenia. Renal toxicity was rare, mild, and transient, observed with 4 (4%) grade I/II events. Ambulatory administration of HD-MTX at 3 g/m2 without MTX clearance monitoring is safe with strict medical guidance. It requires careful selection of patients before administration, and a renal and hepatic monitoring after the administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CNS relapse is a serious event in patient with aggressive non-Hodgkin lymphoma (NHL) and associated with poor outcomes. CNS relapses in DLBCL occurs in 1 to 31% depending on the series and risk factors studied [1, 2]. In peripheral T cell lymphoma, the risk of CNS relapse has not been extensively studied and was estimated between 2.1 and 6.4% in two large retrospective series [3, 4].
The incidence of CNS relapses in both brain parenchyma and meninges is usually observed during the first 2 years of follow-up in DLBCL [5, 6]. The best strategy for preventing CNS relapse is still a matter of debate [7], in all subtypes of non-Hodgkin’s lymphoma, in particular in DLBCL. The value of prophylactic intrathecal chemotherapy is controversial since CNS relapses occur more frequently in brain parenchyma than in meninges and may be observed in patients who have received intrathecal chemotherapy [1, 8]. More aggressive CNS prophylaxis such as systemic high-dose methotrexate (HD-MTX) at > 3 g/m2 seems to be the best alternative in this context [9]. This strategy has been developed in the LYSA group since 1989, after an induction regimen including 4 cycles of intensified CHOP for patients with aggressive DLBCL [10]. The validation of the CNS-IPI score by Schmitz et al. [11] in 2016 rendered possible a better identification of patients with a high risk of CNS relapses.
An exhaustive review of available data about CNS prophylaxis highlights the efficiency of HD-MTX as CNS prophylaxis at a dose superior or equal to 3 g/m2 [12, 13]. HD-MTX administration (usually between 3 and 8 g/m2) is used for a variety of pediatric and adult cancers including osteosarcoma, acute lymphoblastic leukemia, and primary or secondary CNS lymphoma.
MTX is an antimetabolite-targeting folate metabolism and penetrates through cell membranes, particularly at doses where it crosses the blood-brain barrier. MTX is mainly bound (50 to 80%) to albumin in the plasma circulation and its essentially renal clearance explains the possible occurrence of severe toxicity after high-dose administration (> 500 mg/m2). When patients experience delayed MTX elimination, the prolonged exposure to toxic MTX concentrations can lead to significant morbidity. All of these toxicities may lead to non-reversible adverse events and mortality [14]. Regarding renal toxicities, HD-MTX can induce an acute tubular necrosis and precipitate with its metabolite in 2–4% of cases. This is a major complication of HD-MTX that may be reduced by using an antidote, the glucarpidase or carboxypeptidase G2. An increase of more than 50% in serum creatinine 36–48 h after administration of HD-MTX is considered to be predictive of a delay in the elimination of methotrexate [15]. Non-renal toxicities include hepatic, hematological, gastrointestinal, and neurological toxicities. Although severe hepatic cytolysis is rare, a simple increase of liver enzymes is frequently observed, which is usually transient and spontaneously reversible. Superficial ulcers and mucositis can affect the entire digestive tract. Neurological complications may arise because MTX interferes with transmethylation reactions which are crucial for the production of myelin. They may be either acute, immediately after treatment (3.8 to 7.8% in pediatric ALL patients) [16, 17] (mainly leukoencephalopathy) and most of the time reversible, or delayed with neurological and progressive cognitive impairment (necrotic leukoencephalopathy). MTX may also induce hematological toxicity. A study of an elderly population treated with HD-MTX for PCNSL reported 39% grade III/IV neutropenia, 16% grade III/IV anemia, and 6% grade III/IV thrombopenia [18]. Immunoallergic pneumonia leading to pulmonary fibrosis can be observed in very rare cases [19].
Selection of patients on clinical and biological features, clinical monitoring, hydratation urine alkalinization, and leucovorin rescue are associated with an improvement of morbidity and mortality related to HD-MTX [19]. An adapted patient selection and management of systemic HD-MTX administration is required [19]. Because of the risks associated with HD-MTX, most institutions in the world still require a minimum 72-h inpatient stay for administration and monitoring of serum concentrations of MTX. These hospitalizations reduce life quality of patients, and are associated with a significant cost. Several studies, especially in pediatric populations [20,21,22], have provided evidence that outpatient administration of HD-MTX represents a safe modality, on the condition that home intravenous hydration is administered. Few data are available for adult lymphoma populations with the indication of CNS prophylaxis. Recently, Pampin et al. reported an outpatient administration of HD-MTX with daily hospital visits to monitor creatinine value, pH level, and methotrexate levels at 24 h, 48 h, and 72 h [23].
This urged us to report our experience of an outpatient administration of HD-MTX as CNS prophylaxis without MTX clearance monitoring, but based on a careful monitoring of renal and hepatic functions and a strict selection of patients.
The aim of this study was to retrospectively analyze the procedure of an ambulatory administration of HD-MTX for CNS prophylaxis in first-line treatment, based on the renal and hepatic monitoring in a highly selected population of patients with aggressive lymphoma.
Patients and methods
Population
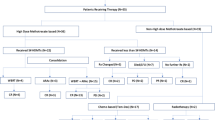
We performed a retrospective analysis of HD-MTX administration in the outpatient clinic among patients with aggressive B cell (n = 98) or T cell (n = 5) non-Hodgkin’s lymphoma (NHL) between January 2013 and December 2016 at Saint-Louis Hospital, Paris, France. All these patients were treated as first-line treatment with CHOP or ACBVP, in association with anti-CD20 for B cell lymphoma and were eligible for HD-MTX to benefit of CNS relapses prophylaxis [11]. CNS relapse prophylaxis was administrated to all patients with aaIPI ≥ 1 in (R)-ACBVP arm of treatment and as assessed by the practitioner for patients receiving (R)-CHOP based on known risk factors.
In the (R)-CHOP group (8 patients), HD-MTX was administered 21 days after the 4th (5 patients), or 6th cycle (3 patients) of R-CHOP − 375 mg/m2 rituximab, 50 mg/m2 doxorubicin, 750 mg/m2 cyclophosphamide, 1.4 mg/m2 vincristine (up to a maximum dose of 2 mg) on day 1, and 60 mg/m2 prednisone on days 1–5. In the (R)-ACVBP group (95 patients), the 4 cycles consisted of an induction part, each cycle containing 375 mg/m2 rituximab if B cell NHL, 75 mg/m2 doxorubicin, and 1200 mg/m2 cyclophosphamide on day 1; 2 mg/m2 vindesine and 10 mg bleomycin on days 1 and 5; and 60 mg/m2 prednisone on days 1–5. CNS prophylaxis was included in the sequential consolidation part, starting 4 weeks after completion of the fourth cycle of R-ACVBP, consisting of 2 cycles of MTX (3 g/m2), with four subsequent cycles of rituximab (375 mg/m2) combined with etoposide (300 mg/m2) and ifosfamide (1500 mg/m2).
All procedures performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Selection criteria for a HD-MTX infusion in outpatient clinic
Our strategy for ambulatory HD-MTX administration was to monitor renal and hepatic functions only and not MTX clearance, as this MTX clearance is not available in the outpatient setting. We then carefully selected the patients based on 6 criteria. These 6 mandatory criteria were (1) patient younger than 60 years; (2) performance status of 0 or 1 at the HD-MTX time infusion; (3) normal renal ≥ 60 ml/min and hepatic functions in the 7 days prior to HD-MTX; (4) albumin level strictly greater than 34 g/l; (5) an absence of significant weight loss (less than10% compared to baseline); and (6) a good understanding of practical medical guidance such as oral hydratation at 2 l of alkaline water per day for 3 days, discontinuation of all drugs with potential for interaction with MTX [24], and guidelines for oral calcium folinate administration after infusion. The selection criteria for patients, in this population with a prophylactic indication, were based on the existing literature on the subject with the aim of a minimal toxicity risk [19, 25, 26].
Treatment administration
The HD-MTX infusion was managed with 3 well-defined periods: the period before infusion, the period during infusion, and the period after the infusion of the MTX. On the day before the HD-MTX infusion, patients were asked to initiate at home the urine alkalinization by drinking 2 l of alkaline water per day, as Vichy St Yorre, as well as during the 24 h following infusion. Cotrimoxazole was discontinued 2 days before HD-MTX until the end of calcium folinate administration. On the day of infusion, a 14% sodium bicarbonate solution was administered to obtain a urine pH > 7.5 within 1 h after administration. If necessary (pH > 7.5 not obtained after 1 h), we pursued alkalinization by increasing the infusion rate. Ondansetron was administered immediately before the infusion. Then, the HD-MTX was infused over a period of 2 h and followed by 1 l of 14% sodium bicarbonate over 1.5 h. After the HD-MTX infusion, the patient started the first dose of oral calcium folinate at the dose of 50 mg at H24 and pursued this treatment every 6 h during 3 days (until day 4) for a total of 12 administrations. An alkaline hyperhydratation with 2 l of alkaline water per day was also maintained during 24 h after administration. Biological analysis of creatinine clearance, ALT, AST was performed 48 h after the administration of HD-MTX and additional tests including a blood count; complete hepatic biology including ALT, AST, gamma GT, and phosphatase alkaline; and creatinine clearance was programmed once a week until the next cycle of chemotherapy. In case of increase in renal function greater than 50%, hepatotoxicity grade ≥ II, or clinical grade ≥ 2 reported toxicity (such as nausea, mucositis), the patient was contacted by the unit for inpatient hospitalization. In this procedure, MTX plasma levels were not monitored as renal and hepatic functions were precisely controlled after HD-MTX infusion (Fig. 1).
Data collection and statistical analysis
The characteristics of the patients collected included the comorbidities and the clinical and biological characteristics at lymphoma diagnosis (sex, age, histology according to the 2016 WHO classification [27], ECOG performance status (PS), Ann Arbor stage, LDH level, extranodal sites, age-adjusted International Prognostic Index (aaIPI), CNS-IPI, induction treatment). The type of toxicities per organ (renal, hepatic, hematological, skin and mucosa, digestive) and grades of toxicities were collected according to the according to Common Terminology Criteria for Adverse Events (CTCAE 5.0), after each infusion, the first and the second infusion, of HD-MTX.
The objective of the study was to evaluate the toxicities with a HD-MTX administration in the outpatient clinic, in terms of incidence and grade per organ. All analyses were performed using Excel software.
Results
Characteristics of the patients
Characteristics of the 103 patients treated with HD-MTX in outpatient clinic are summarized in Table 1. The median age was 41 years old (range = 17; 60). The sex ratio was 1.4 with a larger proportion of males.
Before the initiation of HD-MTX, comorbidities and usual treatments of patients were reviewed in order to determine a potential predisposition to known toxicities. At diagnosis, 31 patients (30.1%) presented comorbidities. These included 7 arterial hypertension, 3 prior history of solid tumors (1 thyroid adenocarcinoma and 2 basal cell carcinoma), 3 type 2 diabetes (1 insulino-requiring—2 non insulino-requiring), 2 psychiatric disorders (1 anorexia and 1 depression), 2 pulmonary diseases (1 asthma and 1 sleep apnea syndrome), 3 non-active chronic viral infections (2 chronic hepatitis B, 1 chronic hepatitis C), and 11 other diseases (3 patients with glaucoma, 3 patients with hypothyroidism, 2 patients with psoriasis, 1 patient with thromboembolic disease, and 1 patient with endometriosis). Three patients presented 2 comorbidities. All the patients had normal hepatic and renal function at time of HD-MTX infusion.
A majority of patients (n = 80, 78% patients) did not receive any concomitant treatment. Two patients received an antidiabetic treatment, 5 an antihypertensive drug, 1 an antiviral treatment, 3 a psychiatric treatment, and 7 others (painkillers, hormonal treatments, iron, thyroid substitution). Five female patients received oral contraception.
Histological subtypes were DLBCL or transformed follicular lymphoma for 79 patients (77%), PMBL for 19 patients (18%), T cell lymphoma (NOS or ALK anaplastic lymphoma) for 5 patients (5%). At presentation most of the patients had a good performance status (n = 92, 89%), a disseminated stage (n = 85, 82.5%), and elevated LDH levels (n = 65, 63%). The age-adjusted IPI (aaIPI) was 2–3 for 54 patients (52%). The CNS-IPI score was retrospectively calculated. A low risk (score 0–1) was found in 40 (39%) patients, an intermediate risk (score 2–3) in 53 (51%), and a high risk (score ≥ 4) in 10 (10%) patients.
Regarding the chemotherapy induction regimen, 95 (92%) patients received an ACVBP associated with anti-CD20 for 92 patients (89%) (rituximab, n = 89 or obinutuzumab, n = 3), and 8 (8%) a CHOP associated with anti-CD20 for 6 patients. Based on Cheson criteria 2014 [28], the response assessment at the end of the standard chemotherapy induction was a complete response for 91 (88%) patients and a partial response for 12 (12%). During induction, all the patients received prophylactic antibiotics (cotrimoxazole-atovaquone in all cases, except 1 who received atovaquone alone) and antiviral treatment (valaciclovir).
Incidence of toxicities
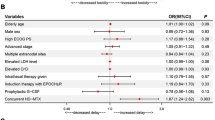
Among the 103 patients, 110 toxicities of any grade were reported during the 2 courses of HD-MTX chemotherapy for a total of 195 cycles. Among these toxicities, 92 (84) were grade I/II and 18 (16%) grade III/IV. Thirty-three (32%) patients presented no toxicity.
Most of the toxicities occurred after cycle 1 (n = 78 toxicities, 71%), including 67 toxicities grade I/II, and 11 grade III/IV, and 32 after cycle 2 including 25 grade I/II and 7 grade III/IV (Fig. 2).
Toxicities per organs
Hepatic toxicity was the most frequent, occurring in 36 patients (35%); this toxicity was mainly an increase in blood liver enzymes. Among them, 14 toxicities were grade I, 14 grade II, and 8 grade III. There were no grade IV hepatic toxicities. Hepatic toxicity occurred mainly after the 1st cycle (32/36, 89%). Four patients presented hepatic toxicity after both 2 cycles, including 1 that presented grade III hepatic toxicities after both. All hepatic toxicities were reversible in less than 15 days.
Skin and mucosal toxicities including mucositis and conjunctivitis (n = 4) occurred in 11 patients (11%) with 8 grade I toxicities, 3 grade II toxicities, and no grade III/IV toxicities, mainly after the first cycle (9/11).
Digestive toxicities mainly included nausea, vomiting, diarrhea, and constipation. Fifteen (15%) patients presented a digestive toxicity with a total of 18 events. Digestive toxicity concerned 16 grade I, 1 grade II, and 1 grade III toxicities (reflex ileus). This occurred either after cycle 1 (9/18, 50%), or after cycle 2 (9/18, 50%). Three patients presented digestive toxicities after cycle 1 and 2.
Twenty-three patients (22%) presented a hematological toxicity with a total of 26 events, either after cycle 1 (14 events) or after cycle 2 (12 events). Seventeen toxicities were grade I/II (7 neutropenia grade I/II, 8 anemia grade I/II, and 2 thrombopenia grade I/II) and 9 were grade III/IV (8 neutropenia with only 1 grade IV and 1 patient with grade III anemia). Three patients presented a cytopenia after both cycles 1 and 2 and 1 patient presented a bicytopenia after cycle 1.
Transient grade I paresthesia occurred in 9 patients (9%) mainly after cycle 1 (7/9, 78%) patients. There was no grade III/IV neurological toxicities reported.
Renal toxicity occurred in 4 patients (3.9%) and was grade I in 2 patients and grade II in 2 patients and after the first cycle for 3 of the 4 patients. There was no grade III/IV renal toxicity.
The other toxicities included arthralgia grade I (1 patient), edema grade I (1 patient), and dyspnea grade II (1 patient).
Overall, only 2 patients (2%) were hospitalized after systemic HD-MTX. The reasons were a grade III reflex ileus (3-day hospitalization) for one patient, and one because of hepatic cytolysis (24-h hospitalization).
CNS relapses in the population
Among the 103 pts. of our study, 78 pts. remained in CR after 2 years of follow-up (76%). Five pts. in CR at the end of treatment were lost, with no follow-up. Twenty patients presented a relapse with 5 CNS relapses (4.9%) (1 was parenchymal, 1 ocular relapse, 3 leptomeningeal relapses) and 15 others relapses (15%). Three CNS relapses occur less than 6 months after the end of treatment, 1 at 1 year of follow-up, and 1 after 3 years.
Ambulatory HD-MTX administration
All patients except for 11 (89%) received the 2 cycles of systemic HD-MTX as an ambulatory administration. Among these 11 patients, 7 received only 1 cycle of HD-MTX because of toxicities and 4 because of lymphoma progression (3 patients) and viral infection (1 patient). The toxicities that induced an arrest of treatment was a grade I/II renal toxicity in 2 patients, a cytolysis in 2 patients (grade III cytolysis for 1, grade II for 1), a grade III neutropenia in 1 patient, and a grade III digestive toxicity in 1 patient.
Discussion
Results of our study highlighted the safety of outpatient HD-MTX administration associated with renal and hepatic monitoring only, in a highly selected population of patients aged less than 60. Overall, 89% of the patients completed the 2 cycles of HD-MTX, and 80% of the patients presented no toxicity or grade I/II toxicities. These results can be compared to other studies with patients with primary CNS lymphoma [26], or CNS prophylaxis with conventional hospitalization [29] in DLBCL patients. Other studies in pediatric osteosarcoma cohort with outpatient MTX administration showed higher level of grade III/IV toxicity, especially neutropenia (18% vs 8% in our cohort) and hepatic cytolysis (39% vs 8% in our cohort). However, the dose of HD-MTX administered in osteosarcoma is much higher, at 12 g/m2 in these series [30]. Only one other recent study [23] reported results on 49 de novo DLBCL outpatients receiving HD-MTX with a similar profile of toxicities. Authors reported no grade III/IV renal failure, in keeping with our observations. Also, 8% of neutropenia was reported which is in exact accordance with our study. Methotrexate serum concentrations were monitored daily starting 24 h after administration until clearance (level ≤ 0.1 μmol/l). Pampin et al. reported no MTX accumulation and no need for intensification of the rescue regimen. Our study, with a larger population (n = 103), supports a safe outpatient administration of HD-MTX in a highly selected population of patients, without MTX clearance monitoring, but with a very strict and careful monitoring of the renal and hepatic functions. Kidney function is a good indicator to monitor proper elimination of methotrexate [31] and should be monitored at 48 h and on day 7.
A key aspect of our work was to identify the selected population of patients eligible for an outpatient administration of H-MTX with no MTX clearance monitoring. This type of surveillance based on renal and hepatic monitoring is a novelty and requires a careful selection of the patient population. Young age less than 60, an albumin level ≥ 35 g/L, a good performance status (0–1), and a stable weight (< loss of 10% compared to baseline) are key parameters to avoid MTX clearance abnormalities [19, 32]. As usually required, a normal renal function, with a clearance ≥ 60 ml/min, is needed for a MTX clearance with no accumulation and subsequent complications. Finally, it is necessary to ensure the patient’s good understanding of practical medical guidance and compliance with the associated rules to allow the safety of outpatient treatment.
The number of any grade toxicity has been observed to be more frequent after cycle 1 than after cycle 2 of HD-MTX. This can be explained by the cumulative toxicity of prior cycles of immunochemotherapy during the induction period. A majority of patients received 4 cycles of R-ACBVP or 4 to 6 cycles of R-CHOP before the HD-MTX administration. Toxicity may be cumulative during this first cycle, especially for hematological and mucositis toxicities [10, 33]. To support this idea of cumulative toxicities after-ACVBP, Fitoussi et al. reported the toxicities after 4 cycles of R-ACVBP and highlighted the hematological (95% vs 43% grade III/IV neutropenia, 59% vs 14% anemia grade III/IV) and mucosal (30% vs 3% grade III/IV) toxicities of R-ACVBP regimen compared to R-CHOP [34, 35]. Only 11 patients did not receive the second cycle of HD-MTX. As this treatment is prophylactic, we recommended declining the second administration in case of any grade III–IV toxicity after the first infusion to avoid any cumulative toxicity.
DLBCL is a very aggressive lymphoma and most patients had a poor quality of life during the treatment, often related to repeated hospitalizations. Thanks to outpatient administration of HD-MTX, patients spent more time at home with a lower impact on their social functioning. Younger patients with DLBCL presented worse quality of life scores than more elderly patients, mainly because of social functioning alterations [36, 37]. Shorter hospitalizations are expected to be a significant factor to improve patient quality of life during the period of therapy.
In conclusion, we demonstrated that HD-MTX outpatient administration based on renal and hepatic monitoring only was feasible and safe in a selected population of patients. The selection is based on very practical and simple criteria. The organization necessary for this treatment can be easily adapted to different hospital settings.
References
Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M (2009) CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 113:3896–3902. https://doi.org/10.1182/blood-2008-10-182253
Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, Lederlin P, Coiffier B (2004) Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol Off J Eur Soc Med Oncol 15:129–133
Gurion R, Mehta N, Migliacci JC, Zelenetz A, Moskowitz A, Lunning M, Moskowitz C, Hamlin P, Horwitz S (2016) Central nervous system involvement in T-cell lymphoma: a single center experience. Acta Oncol 55:561–566. https://doi.org/10.3109/0284186X.2015.1118656
Chihara D, Fanale MA, Miranda RN, Noorani M, Westin JR, Nastoupil LJ, Hagemeister FB, Fayad LE, Romaguera JE, Samaniego F, Turturro F, Lee HJ, Neelapu SS, Rodriguez MA, Wang M, Fowler NH, Davis RE, Medeiros LJ, Oki Y (2018) The risk of central nervous system relapses in patients with peripheral T-cell lymphoma. PLoS One 13:e0191461. https://doi.org/10.1371/journal.pone.0191461
Gleeson M, Counsell N, Cunningham D, Chadwick N, Lawrie A, Hawkes EA, McMillan A, Ardeshna KM, Jack A, Smith P, Mouncey P, Pocock C, Radford JA, Davies J, Turner D, Kruger A, Johnson P, Gambell J, Linch D (2017) Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol 28:2511–2516. https://doi.org/10.1093/annonc/mdx353
Shimazu Y, Notohara K, Ueda Y (2009) Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol 89:577–583. https://doi.org/10.1007/s12185-009-0289-2
Savage KJ (2017) Secondary CNS relapse in diffuse large B-cell lymphoma: defining high-risk patients and optimization of prophylaxis strategies. Hematol Am Soc Hematol Educ Program 2017:578–586. https://doi.org/10.1182/asheducation-2017.1.578
Tomita N, Takasaki H, Ishiyama Y, Kishimoto K, Ishibashi D, Koyama S, Ishii Y, Takahashi H, Numata A, Watanabe R, Tachibana T, Ohshima R, Hagihara M, Hashimoto C, Takemura S, Taguchi J, Fujimaki K, Sakai R, Motomura S, Ishigatsubo Y (2015) Intrathecal methotrexate prophylaxis and central nervous system relapse in patients with diffuse large B-cell lymphoma following rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 56:725–729. https://doi.org/10.3109/10428194.2014.931953
Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, Attal M, Fillet G, Guettier C, Molina TJ, Gisselbrecht C, Reyes F, Groupe d’Etude des Lymphomes de l’Adulte (2003) Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood 102:4284–4289. https://doi.org/10.1182/blood-2003-02-0542
Récher C, Coiffier B, Haioun C, Molina TJ, Fermé C, Casasnovas O, Thiéblemont C, Bosly A, Laurent G, Morschhauser F, Ghesquières H, Jardin F, Bologna S, Fruchart C, Corront B, Gabarre J, Bonnet C, Janvier M, Canioni D, Jais JP, Salles G, Tilly H (2011) Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet 378:1858–1867. https://doi.org/10.1016/S0140-6736(11)61040-4
Schmitz N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH, Glass B, Scott DW, Gascoyne RD, Connors JM, Ziepert M, Pfreundschuh M, Loeffler M, Savage KJ (2016) CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol Off J Am Soc Clin Oncol 34:3150–3156. https://doi.org/10.1200/JCO.2015.65.6520
Cheah CY, Herbert KE, O’Rourke K et al (2014) A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 111:1072–1079. https://doi.org/10.1038/bjc.2014.405
Holte H, Leppä S, Björkholm M, Fluge Ø, Jyrkkiö S, Delabie J, Sundström C, Karjalainen-Lindsberg ML, Erlanson M, Kolstad A, Fosså A, Østenstad B, Löfvenberg E, Nordström M, Janes R, Pedersen LM, Anderson H, Jerkeman M, Eriksson M (2013) Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol 24:1385–1392. https://doi.org/10.1093/annonc/mds621
Aquerreta I, Aldaz A, Giráldez J, Sierrasesúmaga L (2002) Pharmacodynamics of high-dose methotrexate in pediatric patients. Ann Pharmacother 36:1344–1350. https://doi.org/10.1345/aph.1A446
Ramsey LB, Balis FM, O’Brien MM et al (2017) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23:52–61. https://doi.org/10.1634/theoncologist.2017-0243
Mahoney DH, Shuster JJ, Nitschke R et al (1998) Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a pediatric oncology group study. J Clin Oncol 16:1712–1722. https://doi.org/10.1200/JCO.1998.16.5.1712
Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, Krull KR, Inaba H, Rubnitz JE, Metzger ML, Howard SC, Ribeiro RC, Cheng C, Reddick WE, Jeha S, Sandlund JT, Evans WE, Pui CH, Relling MV (2014) Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol Off J Am Soc Clin Oncol 32:949–959. https://doi.org/10.1200/JCO.2013.53.0808
Fritsch K, Kasenda B, Schorb E, Hau P, Bloehdorn J, Möhle R, Löw S, Binder M, Atta J, Keller U, Wolf HH, Krause SW, Heß G, Naumann R, Sasse S, Hirt C, Lamprecht M, Martens U, Morgner A, Panse J, Frickhofen N, Röth A, Hader C, Deckert M, Fricker H, Ihorst G, Finke J, Illerhaus G (2017) High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 31:846–852. https://doi.org/10.1038/leu.2016.334
Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21:1471–1482. https://doi.org/10.1634/theoncologist.2015-0164
Mahadeo KM, Santizo R, Baker L, Curry JO’H, Gorlick R, Levy AS (2010) Ambulatory high-dose methotrexate administration among pediatric osteosarcoma patients in an urban, underserved setting is feasible, safe, and cost-effective. Pediatr Blood Cancer 55:1296–1299. https://doi.org/10.1002/pbc.22772
Zelcer S, Kellick M, Wexler LH, Gorlick R, Meyers PA (2008) The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer 50:1176–1180. https://doi.org/10.1002/pbc.21419
Anderson P (2007) Chemotherapy for osteosarcoma with high-dose methotrexate is effective and outpatient therapy is now possible. Nat Clin Pract Oncol 4:624–625. https://doi.org/10.1038/ncponc0953
Pampín R, Labeaga Y, Rodríguez B, Fernández B, Fernández R, Carbajales M (2019) Experience with ambulatory high-dose methotrexate administration as CNS prophylaxis in patients with non-Hodgkin lymphoma. J Oncol Pharm Pract 1078155219852412:549–555. https://doi.org/10.1177/1078155219852412
(2015) Drug information handbook with international trade names index, 24th edition.. Lexi-Comp Inc, Hudson, Ohio
Ranchon F, Vantard N, Henin E, Bachy E, Sarkozy C, Karlin L, Bouafia-Sauvy F, Gouraud A, Schwiertz V, Bourbon E, Baudouin A, Caffin AG, Vial T, Salles G, Rioufol C (2017) Delayed methotrexate elimination: incidence, interaction with antacid drugs, and clinical consequences? Hematol Oncol 36:399–406. https://doi.org/10.1002/hon.2479
Han X, Ji Y, Ouyang M, Zhu T, Zhou D (2017) Efficacy and safety of HD-MTX based systemic chemotherapy regimens: retrospective study of induction therapy for primary central nervous system lymphoma in Chinese. Sci Rep 7:17053. https://doi.org/10.1038/s41598-017-17359-1
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390. https://doi.org/10.1182/blood-2016-01-643569
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance, Australasian Leukaemia and Lymphoma Group, Eastern Cooperative Oncology Group, European Mantle Cell Lymphoma Consortium, Italian Lymphoma Foundation, European Organisation for Research, Treatment of Cancer/Dutch Hemato-Oncology Group, Grupo Español de Médula Ósea, German High-Grade Lymphoma Study Group, German Hodgkin’s Study Group, Japanese Lymphorra Study Group, Lymphoma Study Association, NCIC Clinical Trials Group, Nordic Lymphoma Study Group, Southwest Oncology Group, United Kingdom National Cancer Research Institute (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3067. https://doi.org/10.1200/JCO.2013.54.8800
Lee K, Yoon DH, Hong JY, Kim S, Lee K, Kang EH, Huh J, Park CS, Lee SW, Suh C (2019) Systemic HD-MTX for CNS prophylaxis in high-risk DLBCL patients: a prospectively collected, single-center cohort analysis. Int J Hematol 110:86–94. https://doi.org/10.1007/s12185-019-02653-7
Villegas Rubio JA, Cacciavillano W, Rose A, Zubizarreta P, Scopinaro M (2017) Ambulatory high-dose methotrexate administration in pediatric osteosarcoma patients at a single institution in Argentina. J Pediatr Hematol Oncol 39:e349–e352. https://doi.org/10.1097/MPH.0000000000000922
Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA (1977) Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med 297:630–634. https://doi.org/10.1056/NEJM197709222971203
Reiss SN, Buie LW, Adel N, Goldman DA, Devlin SM, Douer D (2016) Hypoalbuminemia is significantly associated with increased clearance time of high dose methotrexate in patients being treated for lymphoma or leukemia. Ann Hematol 95:2009–2015. https://doi.org/10.1007/s00277-016-2795-7
Ketterer N, Coiffier B, Thieblemont C, Fermé C, Brière J, Casasnovas O, Bologna S, Christian B, Connerotte T, Récher C, Bordessoule D, Fruchart C, Delarue R, Bonnet C, Morschhauser F, Anglaret B, Soussain C, Fabiani B, Tilly H, Haioun C (2013) Phase III study of ACVBP versus ACVBP plus rituximab for patients with localized low-risk diffuse large B-cell lymphoma (LNH03-1B). Ann Oncol 24:1032–1037. https://doi.org/10.1093/annonc/mds600
Fitoussi O, Belhadj K, Mounier N, Parrens M, Tilly H, Salles G, Feugier P, Ferme C, Ysebaert L, Gabarre J, Herbrecht R, Janvier M, van den Neste E, Morschhauser F, Casasnovas O, Ghesquieres H, Anglaret B, Brechignac S, Haioun C, Gisselbrecht C (2011) Survival impact of rituximab combined with ACVBP and upfront consolidation autotransplantation in high-risk diffuse large B-cell lymphoma for GELA. Haematologica 96:1136–1143. https://doi.org/10.3324/haematol.2010.038109
Coiffier B, Lepage E, Briere J et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242. https://doi.org/10.1056/NEJMoa011795
Oerlemans S, Mols F, Nijziel MR, Zijlstra WP, Coebergh JWW, van de Poll-Franse LV (2014) The course of anxiety and depression for patients with Hodgkin’s lymphoma or diffuse large B cell lymphoma: a longitudinal study of the PROFILES registry. J Cancer Surviv 8:555–564. https://doi.org/10.1007/s11764-014-0367-1
van der Poel MWM, Oerlemans S, Schouten HC, Mols F, Pruijt JFM, Maas H, van de Poll-Franse LV (2014) Quality of life more impaired in younger than in older diffuse large B cell lymphoma survivors compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol 93:811–819. https://doi.org/10.1007/s00277-013-1980-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernard, S., Hachon, L., Diasonama, J.F. et al. Ambulatory high-dose methotrexate administration as central nervous system prophylaxis in patients with aggressive lymphoma. Ann Hematol 100, 979–986 (2021). https://doi.org/10.1007/s00277-020-04341-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04341-7