Abstract
In recent times, there has been a growing interest in understanding the impact of gender on disease biology and clinical outcomes in Philadelphia-negative chronic myeloproliferative neoplasms. Among those, polycythemia vera (PV) is characterized by increased thrombotic risk, systemic symptoms, and overall reduced survival. Here, we aim to summarize data on whether and to what extent female sex can affect PV biology and outcome. To this end, we will discuss the latest acquisitions in terms of pathogenesis, diagnosis, epidemiology, clinical presentation and symptoms burden, thrombotic risk and related treatment strategies, and prognosis in female patients affected by PV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycythemia vera (PV) is included among the Philadelphia-negative chronic myeloproliferative neoplasms (MPNs), together with essential thrombocythemia (ET) and primary myelofibrosis (PMF) [1]. PV and ET could evolve into post-PV or post-ET myelofibrosis (PPV/PET-MF), also known as secondary myelofibrosis (SMF) [2]. PV is more common than MF [3]. It is characterized by excessive red cell production and the release of pro-inflammatory cytokines [4]. These events are secondary to the hyper-activation of the JAK-STAT pathway, caused by mutations in Janus kinase 2 (JAK2) gene [4]. Clinical phenotype is dominated by systemic symptoms and microvascular disturbances; in 30% of cases, splenomegaly may be detected [5, 6]. In the long term, what really affects outcome is the increased risk of thrombosis, evolution into PPV-MF, or into blast phase (BP) [7].
Gender-based discrepancies have been observed in terms of incidence, response to therapies, and prognosis in several solid and hematological cancers [8]. Sex differences may depend on multiple factors, including differentially activated genetic/molecular patterns, immune system function, sex hormones expression [9], and drug metabolism [10]. In solid cancers, as in acute leukemia, incidence, and mortality rates are higher in males. Overall mortality depends not only on multiple factors, including cardiovascular risk factors (which are generally more frequent in males), but also on genetic polymorphisms involved in facilitating carcinogenesis [11]. Besides, increased predisposition to leukemia in males could depend on the lack of estrogens [12]. The latter are supposed to inhibit the Nuclear Factor kappa B (NF-κB) pathway, which regulates the transcription of Interferon Regulatory Factor 4 (IRF4) and, as a consequence, a correct differentiation of B and T cells [12]. Looking at the treatment effect, the anti-CD20 monoclonal antibody rituximab (used in several B lymphoproliferative disorders) seems to achieve better results in females [13, 14]. This is probably due to a faster drug clearance in men and to a different pharmacokinetics secondary to fat tissue abundancy in females [13, 14]. For the same reason, women present greater adverse effects on most anticancer therapies [15,16,17,18].
Despite growing evidence in other cancers, information on gender effect in MPNs is scarce. To understand whether and to what extent female sex could have an impact on PV biology and clinics, a detailed search of the literature was conducted using PubMed (US National Library of Medicine and the National Institutes of Health) and Web of Science (Thomas Reuters Online Academic Citation Index), with publication dates ranging from 1956 to March 2020. To ensure an extensive range of publications were identified, broad search terms for PV, sex, gender, and clinical/epidemiological variables (e.g., incidence, prevalence, frequency, diagnosis, pathogenesis, thrombosis, complications, survival, outcome) were utilized with the addition of alternative spellings and umbrella terms, e.g., erythremia and poliglobulia. Furthermore, we reviewed the literature cited in the identified papers.
Based on this research, we summarize the available data on the impact of female gender in light of the latest acquisitions on PV pathogenesis, diagnosis, epidemiology, clinical presentation, symptom burden, thrombotic risk, prognosis, and treatment strategies.
Diagnostic criteria for PV are tailored on sex
In prepubescents, no sex difference is evident for hemoglobin (Hb) or hematocrit (HCT) levels [19]. On the contrary, during puberty, and in adults, a clear discrepancy emerges, as Hb level is on average 12% higher in males [19]. Androgens play a major role in ruling Hb value and plasma volume, even if the exact mechanism is not fully understood yet [20, 21]. Indeed, in adulthood testosterone is secreted in different concentrations between genders: levels are below 2 nmol/L in women, whereas its production rises to 20-fold and results 15-fold higher in men than in females [22]. Despite the latter are known to have reduced iron storages [23], sex effect on Hb/HCT seems to be independent from them [24, 25]. No interaction was also evident between gender and race [26] or circulating erythropoietin (Epo) levels [27].
Considering the physiological differences in Hb levels between genders, also conventional criteria for defining PV indicated higher threshold values in men (Table 1) [1, 28,29,30,31,32,33,34,35]. The updated World Health Organization (WHO) 2016 diagnostic criteria retained sex-based Hb levels but narrowed considerably the threshold between genders (Table 2) [1]. These changes were made to perform diagnosis earlier: recent studies have shown that patients with JAK2 mutated thrombocytosis and moderately high HCT have increased thrombotic risk (“masked” PV), regardless of sex [36].
Together with Hb or HCT values, the presence of JAK2 mutation and a compatible marrow histology establish nowadays PV diagnosis, even in the absence of reduced Epo levels [1]. Leukocytosis, thrombocytosis, and splenomegaly may provide useful clues. An important exception is PV patients being diagnosed because of splanchnic vein thrombosis (SVT) [37]. The latter are more frequently women and may present significant discrepancies between Hb and HCT levels, due to plasma volume expansion [37, 38].
Incidence of PV is lower in females?
Among various registries, the incidence of PV ranges from 0.4 to 2.8 × 105 per year [39]. Prevalence is 1/3300 people, considering the long life expectancy [39]. PV is a disease occurring mainly in the sixth decade [40] but can be diagnosed at any age. In some publications, it is reported that the risk of myeloid diseases is higher in young females, while in advanced ages it is overexpressed in males [41, 42]. More recently, 426 PV cases followed between 2001 and 2011 were extracted from the SEER (Surveillance, Epidemiology, and End Results) cancer registry [43]. Incidence rate increased significantly in patients aged over 50 years, and even more over 75 [43]. In all age groups, PV risk resulted lower among women [43]. These results parallel those from the ECLAP (European Collaboration on Low-dose Aspirin in Polycythemia Vera), the CYTO-PV (Cytoreductive therapy in PV) prospective studies [44, 45] (female rate was 40.5% and 36.8%, respectively), and a study on 70 young PV subjects (30%) [46]. Also, the subsequent SEER analysis from 2001 to 2016 showed a male to female incidence rate ratio of 1.6, which is consistent with data from European registries [39, 47]. These findings seem to indicate that the incidence rates of PV are lower in females, and this difference holds also in lower-age patients [43, 46].
In contrast to this statement, in two longitudinal studies collecting data on 165 Polycythemia Vera Study Group (PVSG)–defined and 3,382,008 WHO-defined patients, women represented 64% and 58% of the population, respectively [48, 49]. Finally, in a systematic meta-analysis, crude annual incidence did not significantly differ between males and females [3].
These epidemiological discrepancies may partially derive from subsequent changes in diagnostic criteria over a relatively short period of time. Also, MPNs are not always diagnosed in hospital settings and histopathological confirmation is not always performed. This might lead to an inaccurate case ascertainment by registries. On the other hand, claims-based analyses are prone to code errors and lack a validation process [50]. Additionally, the potential impact and changes over time of lifestyle habits on PV incidence may be relevant and requires further investigation. Very recently, MPN incidence was found to be closely related to smoking habits [51] which is generally more common in men.
Finally, selection bias may represent a major issue. For example, a highly different portion of females is enrolled in longitudinal studies (i.e., those collecting data from selected institutions or providing most prognostic information) compared with the fraction involved in interventional trials. A systematic comparison of patient characteristics in clinical vs longitudinal studies may demonstrate this bias’s relevance on the heterogeneity of results and on the quality of the final evidence.
PV pathogenesis: A different genetic background between genders
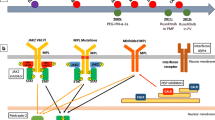
JAK2 mutation involves exon 14 (JAK2V617F, from now on JAK2) in 95–99% of PV patients and it is homozygous in around 30–50% of cases. The other 1–5% of subjects carry “gain of function” mutations in exon 12 [5, 52, 53]. The latter showed an association with younger age or higher Hb level, while no gender imbalance was identified in two independent cohorts [54, 55], suggesting that sex does not play a key role in exon 12 genetic instability.
At diagnosis, female PV patients present less frequently with a homozygous JAK2 genotype compared with males (median: 61% vs 80%). This is consistent with a lower JAK2 variant allele frequency (VAF) in the former, even adjusting for age and disease duration [56]. During follow-up, JAK2 VAF is similar within the first year from diagnosis, but it becomes significantly higher in men (median: 82% vs 63%) when evaluated after at least eight years. A possible explanation is that a lower JAK2 VAF induces fewer mitotic recombination events and less expansion of homozygous JAK2 clones, leading to a predominant distribution within the PV phenotypes [48, 57]. A selective JAK2 expression in platelets has been reported only in women with ET but not PV [56, 58].
In PV, JAK2 mutation is not the only detectable genetic abnormality [59, 60]. Over 50% of patients showed non-driver sub-clonal aberrations in a next-generation sequencing (NGS) study [61]. Within 76 MPN patients sequenced at Johns Hopkins Hospital, females showed fewer additional somatic genetic alterations [62]. On the contrary, males present more frequently with non-driver mutations as ASXL1, SRSF2, U2AF1, EZH2, IDH1, and IDH2 [62]. A Mayo Clinic-Florence collaboration analyzed 906 molecularly annotated MPNs, including 404 PV [53]. In this case, additional genetic abnormalities were found in about 18% of PV patients, without gender differences [53].
Of note, the JAK2 mutation has been detected also in the general population with a frequency of 0.1–0.2% with increasing age and male sex associated with its acquisition. However, individuals who develop the JAK2 mutation in the general population have a VAF below 2% and minimal evidence of MPN [63, 64].
Gene expression profile in circulating CD34+ cells from 19 PV cases has been investigated [65]. Authors found that fewer genes were differentially expressed in females than in males (235 vs 571), but the former had more than 3-fold activated molecular pathways. For instance, the pentose phosphate and the fatty acids pathways were not activated in males [65]. This finding suggests that selected metabolic mechanisms might play a role in the pathogenesis of PV in women [65]. In the same study, 102 genes with differential regulation were concordant between genders [65].
Concerning cytogenetic analysis in PV, around 20% had abnormalities, without sex differences [66, 67].
The inflammatory milieu plays an important role in MPNs. A field of active research is related to extracellular vesicles, which are small particles released by activated cells and involved in intercellular signaling. To date, no difference between men and women was found for the concentration of inflammatory biomarkers, such as high-sensitivity C-reactive protein or pentraxin-3 [68]. Consistently, in a cohort of 38 PV patients, no differences were observed in terms of circulating platelet-derived and tissue factor–positive extracellular vesicles (even though the content was increased in both genders) (Catani L, personal data). Table 3 summarizes peculiarities in epidemiology and the genetic background of PV in females.
PV phenotype in women: A milder clinical presentation with a relevant symptoms burden
The prospective ECLAP trial enrolled 1638 patients and therefore represented a valid tool to analyze clinical characteristics of PV [40]. Disease phenotype was milder in women compared with that in men: at study entry, the former showed lower rates of myocardial infarction (11.3% vs 5.8%) and peripheral arterial disease (6.1% vs 2.9%). This could partially depend on the reduced cardiovascular risk factors (i.e., smoking) incidence among females [69].
Besides, the ECLAP study underlined lower HCT levels (46 ± 6% vs 48 ± 6%) and smaller splenomegalies in women [40]. However, it should be acknowledged that the clinical phenotype of PV, particularly in terms of HCT levels, is significantly influenced by gender-specific diagnostic thresholds (Fig. 1).
Thrombosis history and cardiovascular risk factors according to gender in a cohort of 1638 polycythemia vera patients (adapted by Landolfi et al. [40]). Legend: AMI, acute myocardial infarction; PAT, peripheral arterial thrombosis with residual intermittent claudication; VT, venous thrombosis
Of note, a history of venous thrombosis was more common in females (11.4% vs 7.9%) [40] (Fig. 1). In particular, young women had a significantly increased rate of SVT, including extra-hepatic portal vein thrombosis and Budd-Chiari syndrome [38, 40, 69,70,71,72]. In a cohort of 270 consecutive JAK2-positive MPNs, 15 women had vascular complications at diagnosis: eight of them were affected by PV and had SVT [69]. Of note, this subgroup had lower leukocytosis, JAK2 VAF, and cholesterol levels, along with less smoking history when compared with men [69]. However, mortality in patients with SVT does not seem to depend on gender [72]. A retrospective Spanish study suggested that the excess of fatality in cases of SVT was related to hepatic complications, major bleeding, and second cancers [72]. Recently, retrospective data on 518 MPN-related SVT cases (including 192 PV) have been reported [38]. Female patients were prevalent, reaching 53.1% in PV [38]. The median age at diagnosis was significantly lower in the case of SVT compared with MPNs controls [38]. The peculiar gender and age distribution in MPNs-SVT suggests that traditional risk factors for thrombosis are of limited contribution in this context [38]. The reasons for this distribution are not completely understood. Sex hormones probably play a pathogenic role. Additional risk factors for SVT in females may include the use of oral contraceptive or hormone replacement therapy in women [73,74,75,76]. Also, a hypercoagulable state could increase the risk of SVT [77, 78]. A strong association was reported between the presence of factor V Leiden and the development of Budd-Chiari syndrome or portal vein thrombosis (PVT), whereas prothrombin G20210A mutation was associated only with non-cirrhotic PVT [79].
Gender effect seems particularly important in determining symptoms burden. Out of 2006 MPNs patients that completed the Myeloproliferative Neoplasm-Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) and the Brief Fatigue Inventory Patient Reported Outcome, females were 1089 (54.3%) [80]. The latter had more severe and frequent individual symptoms, along with overall higher TSS [80]. Looking at the symptoms list, those related to abdominal discomfort and microvascular disturbances (i.e., headache, dizziness) were predominant in women [80]. Notably, factors that may contribute to disease burden, like anemia or other adverse prognostic features, were comparable between genders [80]. However, there was not a corresponding decrease in the quality of life in females [80]. Whether this counterintuitive finding depends on the propensity of women to express their complaints, or to a greater capacity of men to tolerate symptoms, or by an overall low sensitivity of the MPN-SAF TSS remains to be clarified.
Gender seems not predictive of thrombotic risk and treatment efficacy in PV
There is consistent evidence that thrombotic risk in PV is not significantly influenced by sex. Of note, in the ECLAP study, this holds true except for a higher incidence of SVT at or near diagnosis in female patients [40]. In the CYTO-PV study, only HCT levels and leukocyte count were significantly associated with increased risk of thrombosis, regardless of sex [45, 81]. The same incidence rate between genders was also found in a large cohort of MPNs prospectively evaluated at the Mayo Clinic, which included 711 PV patients [80]. Therefore, current PV therapeutic recommendations do not stratify vascular risk on sex, but according to age and thrombosis history [4, 82, 83]. Phlebotomies and aspirin are indicated in patients younger than 60 years of age, while cytoreduction is required in “high-risk” patients, that subject older than 60 years and/or with previous thrombosis [4, 82, 83]. Only recently, Karantanos et al. reported the outcome of 694 MPNs patients (394 females, 258 males; 311 ET, 252 PV, and 89 PMF) prospectively followed at the Johns Hopkins Hospital from 2005 to 2019 [62]. They observed that arterial ischemic events were more common in males, independently from driver mutation status and MPN subtype [62].
Regarding treatment [83], a major goal of PV therapy is keeping HCT levels below 45%, in order to reduce thrombotic complications [45]. Prospective trials such as the CYTO-PV study have definitively established that this HCT target is independent of gender [45, 84]. Some have advocated a lower HCT threshold (< 42%) as more appropriate in females [5, 84]. Among the reasons, the fact that, at every body weight, women normal red cell mass is around 600 mL lower than men [5, 84]. Therefore, when HCT is 45%, females have, at least, a blood excess of 600 mL [5, 84]. This difference could in part be explained by a 15-fold lower testosterone levels than men [22].
A recent consensus pointed out that a 40–42% HCT target is appropriate in subjects with persistent or recurrent symptoms of hyper-viscosity despite HCT < 45%, regardless of sex [70]. A stricter threshold seems not indicated even in case of pregnancy, at least in “low-risk” cases [85]. Despite the absence of formal proof, in a recent survey conducted among hematologist members of the MPN Research and Aplastic Anemia and Myelodisplastic Syndromes (MDS) Foundations, almost half reported to apply a gender-dependent approach for the maintenance of HCT in PV [86].
To date, scarce information is available on a possible different safety/efficacy profile of drug treatments in women with PV. In the CYTO-PV trial, 365 PV patients on therapy with phlebotomies, hydroxyurea (HU), or both were randomized to receive either more or less intensive treatment [45]. Reduction of cardiovascular events was higher in the former cohort and independent from sex, as well as age, previous thrombosis, platelet or leukocyte counts, splenomegaly, past cytoreductive treatment, or antiplatelet/anticoagulant drugs [45]. The observational study REVEAL prospectively followed 2510 PV patients in the USA [87]. The reported proportion of females receiving HU was comparable with that of males [87].
In a series of 890 PV cases from the Spanish Registry, Alvarez-Larran et al. investigated the clinical relevance of resistance/intolerance to HU, as defined by the 2009 European Leukemia Net (ELN) criteria [88, 89]. Resistance to HU was recorded in 5.7% of patients and distributed as follows: 3.3% need for phlebotomies, 1.6% uncontrolled myeloproliferation, 0.8% failure to reduce massive splenomegaly [89]. Intolerance to cytoreduction occurred in 10.7% of cases, mainly (9%) due to extra-hematological toxicity [89]. While older age correlated with resistance/intolerance to HU, female gender resulted significantly associated only with time to unacceptable extra-hematological toxicity [89]. Nevertheless, the latter did not increase mortality rate, risk of vascular complications, and of SMF/BP transformation [89].
Ruxolitinib is a JAK1/JAK2 inhibitor that is used as a second-line therapy for PV patients’ resistant or intolerant to HU [90, 91]. Its approval was based on the results of the prospective clinical trials Response and Response-2, which enrolled 222 patients with and 173 without palpable splenomegaly, respectively [90, 91]. Subjects were randomized to receive either ruxolitinib or best available therapy (mainly HU) [90, 91]. The primary endpoint of the Response study was the proportion of patients achieving HCT control and at least a 35% reduction in spleen volume at week 32, while it was only HCT control at week 28 in Response-2 [90, 91]. In both trials, primary efficacy results were consistent across all subgroups, regardless of gender [90, 91]. Notably, also symptom control was comparable [90, 91]. Longer-term follow-up of these studies has not disclosed any significant sex difference in terms of response durability and toxicities [92, 93]. However, these studies had very heterogeneous designs (retrospective and prospective), inclusion criteria (high-risk patients with or without resistance or intolerance to HU), and primary endpoints (cardiovascular death or thrombotic event in the CYTO-PV study, rate of HCT/spleen control in the Response/Response-2 trials).
The controversial role of female sex on PV survival
While a longer overall survival for female patients has been demonstrated in large cohorts of ET [53, 94, 95], the prognostic impact of gender in PV is still a matter of debate.
Many retrospective studies have demonstrated a clear survival disadvantage in patients with PV compared with age- and sex-matched healthy controls [7, 95,96,97]. The International Working Group for Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) prognostic model for PV is the most used and it does not consider gender [97]. Indeed, this score divides patients into three risk categories based on the following demographic/laboratory characteristics: age, previous venous thrombosis, and leukocytosis [97]. Hazard ratio (HR)–weighted adverse points were assigned to age ≥ 67 years (5 points), age 57–66 years (2 points), leukocyte count ≥15 × 109/L (1 point), and venous thrombosis (1 point), to devise a prognostic model that included low-risk (0 points), intermediate-risk (1–2 points), and high-risk (≥ 3 points) categories. The prognostic model stratified between high-risk (median survival = 8.3 years; HR, 11.1; 95% CI: 6.3–19.6), intermediate-risk (median survival = 15 years; HR, 3.5; 95% CI: 1.9–6.2), and low-risk (median survival = 26 years) patient groups [97]. In a retrospective cohort of 831 consecutive MPNs (396 PV and 435 ET), male sex was associated with shorter survival only in ET, but not in PV [95].
Conversely, in 261 PV retrospectively collected in the Spanish registry, men had a 2-fold increase in mortality rate compared with women (HR:2.3, 95% CI: 1.2–4.3, p = 0.008) [98]. Additionally, age > 65 years and leukocytosis >10 × 109/L at diagnosis were associated with a significantly shorter survival [98]. Recently, the Mutation-enhanced International Prognostic Scoring System (MIPSS) was proposed for ET and PV [53]. Of note, male sex had an impact on ET survival (HR 1.8, 95% CI: 1.3–2.4), while it did not retain statistical significance in PV [53].
Evolution into PPV-MF occurs in around 5% and 15% of patients at 10 and 15 years of follow-up, respectively [95]. SMF transformation is associated with a significant worsening of symptoms and quality of life, along with a substantial reduction in survival expectation [2, 95, 99,100,101]. PV could also progress into BP in 3% and 10% of patients after 10 and 20 years from diagnosis, respectively [97]. This eventuality confers a very poor outcome, with a median survival of 1.5 to 2.5 months if not treated [102].
To date, sex is not included among risk factors for PV evolution into PPV-MF and BP. Conventional risk factors for PPV-MF and BP transformation include age ≥ 60 years, leukocytosis (≥ 15–30 × 109/L), homozygosity for JAK2 mutation, and exposures to alkylating agents (reviewed by Cuthbert D et al.) [103,104,105,106,107,108,109,110]. NGS based studies demonstrated also that non-driver mutations in myeloid genes could influence PV evolution: ASXL1, SRSF2, RUNX1, SF3B1, and IDH1/2 for PPV-MF; ASXL1, TP53, SRSF2, IDH1/2, and RUNX1 for BP [110]. In the recent MIPSS-ET/PV study, only 8 (2%) of PV patients showed “unfavorable” genetic alterations. IDH2 and RUNX1 mutations adversely affected BP-free survival (BP-FS) and U2AF1 SMF-FS, when considering the Mayo Clinic cohort. As for Florence database, SRSF2 influenced only BP-FS [53].
Looking at young PV patients, 70 subjects aged less than 50 years were followed for a median time of 14 years in a monocentric cohort [46]. Overall, five (7%) patients developed BP and the same percentage PPV-MF, with a 20-year cumulative risk of 15% and 10%, respectively [46]. Leukocytosis resulted in the only clinical parameter associated with PPV-MF evolution, while other variables as gender did not influence disease progression [46].
The MYSEC (Myelofibrosis Secondary to Polycythemia vera and Essential Thrombocytemia) project is an international collaboration that collected 684 molecularly annotated SMF [101, 111]. In this cohort, ET and PV diagnosis occurred at a younger age in females (median 50 vs 53 years) while age at the time of SMF transformation was similar between genders (median 63 vs 65 years) [111]. Therefore, ET and PV seem to evolve slower in females, as documented by a longer time to progression into SMF compared with men (median 11.3 vs 10.1 years) [111]. Whether this observation is linked to a different disease biology among females, or to a higher propensity of the latter to undergo blood tests and medical evaluation, is still to be determined.
Considering the whole MYSEC SMF dataset, women were characterized by a more indolent phenotype: higher platelet count, smaller splenomegaly, and lower circulating blasts [111]. Besides, they showed a longer life expectancy, with a median survival of 10.1 compared with 8.1 years in males [111]. Actually, looking only at the PPV-MF sub-cohort, gender did not impact neither the above clinical parameters nor mortality [111, 112].
There are anyway some data advocating for a prognostic relevance of sex in PV [96]. In a sub-analysis of the ECLAP study, BP or MDS were diagnosed in 22 patients [96]. Notably, together with age older than 60 years and lower blood cholesterol levels, also female sex was strongly associated with increased risk of BP/MDS progression [96]. In Karantanos et al., male sex is predictive of SMF risk, independently from age, clinical phenotype at diagnosis, and driver mutation status [62]. Besides, the authors found a significant association with BP progression, independently from age at diagnosis and driver mutations [62] (Fig. 2).
Men with MPNs have worse survival, and shorter time to MF transformation compared with females. Reproduced by Karantanos T et al. [62]
Prognostic factors for mortality, evolution into PPV-MF, or into BP in female PV patients are listed in Table 4.
The unique aspects of polycythemia vera in women: Facing reproductive issues
Considering the abovementioned median age at diagnosis, around 15% of PV patients are less than 40 years of age at the time of diagnosis [41]. Therefore, women of childbearing age may suffer from PV, which can also be diagnosed in pregnancy or during investigations for recurrent pregnancy loss. To date, only case reports and small cohorts [113,114,115] are specifically focused on pregnancies in PV patients. More data are available in ET [116].
In 2005, Robinson et al. analyzed 18 pregnancies in 8 women affected by PV: about 65% had a positive outcome, while about 25% developed maternal complications [113]. A more recent Italian paper retrospectively collected 24 pregnancies in 15 PV cases and confirmed a comparable live birth rate (62.5%, of which 41.7% ended with a full term and 20.8% with a preterm delivery) [115]. The incidence of maternal complications was slightly lower (16.7%) [115] and nine (37.5%) pregnancies ended because of fetal loss, which was a late event in 44% cases [115]. In a subsequent series of 11 pregnancies in 7 females with PV, 8 (72.7%) ended with a full-term delivery [117]. In a recent meta-analysis including a total of 779 women and 1226 pregnancies in patients with PV and ET, the live birth rate was higher in MPN patients who received low-dose aspirin during pregnancy than those managed with observation alone (11 studies, 227 patients; unadjusted OR, 8.6; 95% CI, 4.0–18.1, p < 0.001). Also, a trend (albeit not statistically significant) towards further improvement in the live birth rate with the addition of low molecular weight heparin (LMWH) to aspirin was observed (6 studies, 96 pregnancies; unadjusted OR, 2.1 for aspirin with heparin vs aspirin alone; 95% CI, 0.5–9.0, p = 0.30). Finally, the use of IFN with or without aspirin or heparin during pregnancy was associated with a higher live birth rate (6 studies, 90 patients, unadjusted OR, 9.7; 95% CI, 2.3–41.0; p = 0.002) [116]. Despite the limited numbers, maternal morbidity and gestational outcome are similar to ET [116] and secondary to thrombotic events.
In pregnant ET, the role of JAK2 has been debatable, with studies suggesting its correlation with late fetal loss, and others denying this evidence [57, 115, 116, 118, 119]. Even if PV is strictly associated with JAK2, no association has yet been found between pregnancy complications and differential type of mutation (exon 12 vs exon 14) or mutation load (heterozygous vs homozygous).
Indications on gestation management in MPNs are mainly derived from experts’ consensus. The 2011 ELN treatment MPN guidelines state that a pregnancy must be considered at high risk for negative outcome if the patient presents at least one of the following PV-related complications: (1) venous/arterial thrombosis or bleeding (whether pregnant or not); (2) three recurrent first-trimester losses, intrauterine growth restriction, fetal death, stillbirth; (3) severe pre-eclampsia [120]. Besides, placental abruption, significant ante- or postpartum bleeding or platelet count greater than 1.500 × 109/L are associated with increased risk of complications during pregnancy [120]. According to ELN guidelines, all PV cases should be managed with low-dose aspirin until delivery and phlebotomies in order to keep HCT below 45%, at least in low-risk pregnancies [85, 120]. A stricter HCT threshold has been recently advocated in high-risk patients [85, 120]. In addition, prophylactic dose low molecular weight heparin should be given after delivery, until 6 weeks postpartum [120]. In unfavorable cases, patient’s management depends on the type of risk: if there were major thrombosis or severe pregnancy complications, low molecular weight heparin throughout the gestation period should be added. In the case of extreme thrombocytosis or previous major bleeding, it is indicated to avoid low-dose aspirin and consider interferon alfa [120].
More detailed indications emerged from a more recent comprehensive review [121]. Of note, cytoreductive therapy is recommended if already indicated before pregnancy or in the case of a high-risk pregnancy. Although none of the cytoreductive drugs used in MPN have a product license for use in pregnancy, IFN is the drug of choice, since it was never associated with teratogenic effects [122,123,124]. Conversely, fetal abnormalities were reported in humans and animals exposed to HU [125, 126]. Anagrelide may cross the placenta and have the potential to cause fetal thrombocytopenia in pregnancy [127]. As for pre-conceptual planning, it is important to optimize MPN management, to correct cardiovascular risk factors, and counsel women about pregnancy complications. During the gestational period, fetal growth should be regularly monitored by ultrasound. Maternal dehydration and immobility must be avoided. Breast feeding should be planned on an individual basis, considering the treatment needs. Low-dose aspirin seems to be excreted in very small amount in human milk, and the daily use of an 81-mg dose of aspirin should be considered safe during lactation [128].
Finally, the use of combined oral contraceptive either as contraception or to control excessive menstrual bleeding is not appropriate in female PV patients due to risks of venous thrombosis [129]. Other forms of contraception such as the progesterone-only pill, implant, depot contraceptive injection, or intrauterine systems are acceptable [130].
Conclusions
For decades, gender differences in PV were not considered an investigational priority, given the paucity of exploratory tools in this rare disease. In recent years, the need for efficient treatments has led to increased acquisitions in terms of PV pathogenesis, phenotype, and prognosis. In this contest, sex has emerged as a potential disease modifier, despite its role is still controversial under many aspects, including thrombotic risk, and survival. The mechanisms by which sex may influence disease course and its interactions with other environmental and cultural factors remain also to be clarified. However, this review underscores the importance of clinical and translational gender-based research. In the coming years, collecting prospective data on PV patients stratified by sex will probably contribute to the development of personalized treatment strategies.
Data availability
Not applicable.
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405. https://doi.org/10.1182/blood-2016-03-643544
Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, Dupriez B, Levine RL, Passamonti F, Gotlib J, Reilly JT, Vannucchi AM, Hanson CA, Solberg LA, Orazi A, Tefferi A (2008) Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the international working Group for Myelofibrosis Research and Treatment. Leukemia 22(2):437–438. https://doi.org/10.1038/sj.leu.2404914
Titmarsh GJ, Duncombe AS, McMullin MF, O’Rorke M, Mesa R, De Vocht F, Horan S, Fritschi L, Clarke M, Anderson LA (2014) How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 89(6):581–587. https://doi.org/10.1002/ajh.23690
McMullin MF, Harrison CN, Ali S, Cargo C, Chen F, Ewing J, Garg M, Godfrey A, S SK, McLornan DP, Nangalia J, Sekhar M, Wadelin F, Mead AJ (2019) A guideline for the diagnosis and management of polycythaemia vera. A British Society for Haematology guideline. Br J Haematol 184(2):176–191. https://doi.org/10.1111/bjh.15648
Spivak JL (2019) How I treat polycythemia vera. Blood 134(4):341–352. https://doi.org/10.1182/blood.2018834044
Passamonti F (2012) How to manage polycythemia vera. Leukemia 26(5):870–874. https://doi.org/10.1038/leu.2011.334
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, Gangat N, Fjerza R, Belachew AA, Lasho TL, Ketterling RP, Hanson CA, Rambaldi A, Finazzi G, Thiele J, Barbui T, Pardanani A, Vannucchi AM (2014) Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 124(16):2507–2513; quiz 2615. https://doi.org/10.1182/blood-2014-05-579136
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. https://doi.org/10.3322/caac.21332
Ratajczak MZ (2017) Why are hematopoietic stem cells so ‘sexy’? On a search for developmental explanation. Leukemia 31(8):1671–1677. https://doi.org/10.1038/leu.2017.148
Kim HI, Lim H, Moon A (2018) Sex differences in cancer: epidemiology, genetics and therapy. Biomol Ther 26(4):335–342. https://doi.org/10.4062/biomolther.2018.103
Bolufer P, Collado M, Barragan E, Cervera J, Calasanz MJ, Colomer D, Roman-Gomez J, Sanz MA (2007) The potential effect of gender in combination with common genetic polymorphisms of drug-metabolizing enzymes on the risk of developing acute leukemia. Haematologica 92(3):308–314. https://doi.org/10.3324/haematol.10752
Do TN, Ucisik-Akkaya E, Davis CF, Morrison BA, Dorak MT (2010) An intronic polymorphism of IRF4 gene influences gene transcription in vitro and shows a risk association with childhood acute lymphoblastic leukemia in males. Biochim Biophys Acta 1802(2):292–300. https://doi.org/10.1016/j.bbadis.2009.10.015
Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, Wenger M, Nickenig C, Peter N, Lengfelder E, Metzner B, Rixecker T, Zwick C, Pfreundschuh M, Reiser M (2012) The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 119(14):3276–3284. https://doi.org/10.1182/blood-2011-09-380949
Riihijarvi S, Taskinen M, Jerkeman M, Leppa S (2011) Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur J Haematol 86(2):124–128. https://doi.org/10.1111/j.1600-0609.2010.01541.x
Wang J, Huang Y (2007) Pharmacogenomics of sex difference in chemotherapeutic toxicity. Current drug discovery technologies 4(1):59–68. https://doi.org/10.2174/157016307781115485
Tran C, Knowles SR, Liu BA, Shear NH (1998) Gender differences in adverse drug reactions. J Clin Pharmacol 38(11):1003–1009. https://doi.org/10.1177/009127009803801103
Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U, Stocker DN, Braunschweig S, Kullak-Ublick GA, Galeazzi RL, Follath F, Gasser T, Meier PJ (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49(2):158–167. https://doi.org/10.1046/j.1365-2125.2000.00132.x
Anderson GD (2005) Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. Journal of women’s health (2002) 14(1):19–29. https://doi.org/10.1089/jwh.2005.14.19
Murphy WG (2014) The sex difference in haemoglobin levels in adults - mechanisms, causes, and consequences. Blood Rev 28(2):41–47. https://doi.org/10.1016/j.blre.2013.12.003
Shahani S, Braga-Basaria M, Maggio M, Basaria S (2009) Androgens and erythropoiesis: past and present. J Endocrinol Investig 32(8):704–716. https://doi.org/10.1007/bf03345745
Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, Connor Westfall J, Bae H, Gordeuk V, Bhasin S (2014) Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 69(6):725–735. https://doi.org/10.1093/gerona/glt154
Handelsman DJ, Hirschberg AL, Bermon S (2018) Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev 39(5):803–829. https://doi.org/10.1210/er.2018-00020
Cook JD, Flowers CH, Skikne BS (2003) The quantitative assessment of body iron. Blood 101(9):3359–3364. https://doi.org/10.1182/blood-2002-10-3071
Yip R, Johnson C, Dallman PR (1984) Age-related changes in laboratory values used in the diagnosis of anemia and iron deficiency. Am J Clin Nutr 39(3):427–436. https://doi.org/10.1093/ajcn/39.3.427
Vahlquist B (1950) The cause of the sexual differences in erythrocyte hemoglobin and serum iron levels in human adults. Blood 5(9):874–875
Garn SM, Smith NJ, Clark DC (1975) Lifelong differences in hemoglobin levels between blacks and whites. J Natl Med Assoc 67(2):91–96
Tilling L, Hunt J, Jiang B, Sanders TA, Clapp B, Chowienczyk P (2013) Endothelial function does not relate to haemoglobin or serum erythropoietin concentrations and these do not explain the gender difference in endothelial function in healthy middle-aged men and women. Eur J Clin Investig 43(3):225–230. https://doi.org/10.1111/eci.12033
Pearson TC, Messinezy M (1996) The diagnostic criteria of polycythaemia rubra vera. Leukemia & lymphoma 22(Suppl 1):87–93
Vardiman JW, Harris NL, Brunning RD (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100(7):2292–2302. https://doi.org/10.1182/blood-2002-04-1199
Tefferi A, Thiele J, Vardiman JW (2009) The 2008 World Health Organization classification system for myeloproliferative neoplasms: order out of chaos. Cancer 115(17):3842–3847. https://doi.org/10.1002/cncr.24440
Berlin NI (1975) Diagnosis and classification of the polycythemias. Semin Hematol 12(4):339–351
Berk PD, Goldberg JD, Donovan PB, Fruchtman SM, Berlin NI, Wasserman LR (1986) Therapeutic recommendations in polycythemia vera based on polycythemia vera study group protocols. Semin Hematol 23(2):132–143
Murphy S, Peterson P, Iland H, Laszlo J (1997) Experience of the polycythemia Vera study group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol 34(1):29–39
Pierre R, Imbert M, Thiele J et al (2001) Tumours of haematopoietic and lymphoid tissues. IARC Press, London
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114(5):937–951. https://doi.org/10.1182/blood-2009-03-209262
Lussana F, Carobbio A, Randi ML, Elena C, Rumi E, Finazzi G, Bertozzi I, Pieri L, Ruggeri M, Palandri F, Polverelli N, Elli E, Tieghi A, Iurlo A, Ruella M, Cazzola M, Rambaldi A, Vannucchi AM, Barbui T (2014) A lower intensity of treatment may underlie the increased risk of thrombosis in young patients with masked polycythaemia vera. Br J Haematol 167(4):541–546. https://doi.org/10.1111/bjh.13080
Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ (2013) Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood 122(11):1881–1886. https://doi.org/10.1182/blood-2013-06-508416
Sant’Antonio E, Guglielmelli P, Pieri L, Primignani M, Randi ML, Santarossa C, Rumi E, Cervantes F, Delaini F, Carobbio A, Betti S, Rossi E, Lavi N, Harrison CN, Curto-Garcia N, Gisslinger H, Gisslinger B, Specchia G, Ricco A, Vianelli N, Polverelli N, Koren-Michowitz M, Ruggeri M, Girodon F, Ellis M, Iurlo A, Mannelli F, Mannelli L, Sordi B, Loscocco GG, Cazzola M, De Stefano V, Barbui T, Tefferi A, Vannucchi AM (2020) Splanchnic vein thromboses associated with myeloproliferative neoplasms: an international, retrospective study on 518 cases. Am J Hematol 95(2):156–166. https://doi.org/10.1002/ajh.25677
Moulard O, Mehta J, Fryzek J, Olivares R, Iqbal U, Mesa RA (2014) Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 92(4):289–297. https://doi.org/10.1111/ejh.12256
Landolfi R, Di Gennaro L, Nicolazzi MA, Giarretta I, Marfisi R, Marchioli R (2012) Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 7(6):509–515. https://doi.org/10.1007/s11739-011-0634-3
McNally RJ, Rowland D, Roman E, Cartwright RA (1997) Age and sex distributions of hematological malignancies in the U.K. Hematol Oncol 15(4):173–189. https://doi.org/10.1002/(sici)1099-1069(199711)15:4<173::aid-hon610>3.0.co;2-k
Cartwright RA, Gurney KA, Moorman AV (2002) Sex ratios and the risks of haematological malignancies. Br J Haematol 118(4):1071–1077. https://doi.org/10.1046/j.1365-2141.2002.03750.x
Deadmond MA, Smith-Gagen JA (2015) Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973-2011. J Cancer Res Clin Oncol 141(12):2131–2138. https://doi.org/10.1007/s00432-015-1983-5
Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, Barbui T (2004) Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 350(2):114–124. https://doi.org/10.1056/NEJMoa035572
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, De Stefano V, Elli E, Iurlo A, Latagliata R, Lunghi F, Lunghi M, Marfisi RM, Musto P, Masciulli A, Musolino C, Cascavilla N, Quarta G, Randi ML, Rapezzi D, Ruggeri M, Rumi E, Scortechini AR, Santini S, Scarano M, Siragusa S, Spadea A, Tieghi A, Angelucci E, Visani G, Vannucchi AM, Barbui T (2013) Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 368(1):22–33. https://doi.org/10.1056/NEJMoa1208500
Passamonti F, Malabarba L, Orlandi E, Barate C, Canevari A, Brusamolino E, Bonfichi M, Arcaini L, Caberlon S, Pascutto C, Lazzarino M (2003) Polycythemia vera in young patients: a study on the long-term risk of thrombosis, myelofibrosis and leukemia. Haematologica 88(1):13–18
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, KA (eds) C SEER Cancer Statistics Review, 1975–2016. National Cancer Institute B ethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019
Stein BL, Williams DM, Wang NY, Rogers O, Isaacs MA, Pemmaraju N, Spivak JL, Moliterno AR (2010) Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica 95(7):1090–1097. https://doi.org/10.3324/haematol.2009.014407
Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, Roncoroni E, Astori C, Merli M, Boggi S, Pascutto C, Lazzarino M, Cazzola M (2010) A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 24(9):1574–1579. https://doi.org/10.1038/leu.2010.148
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM (2019) Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev 36:70–87. https://doi.org/10.1016/j.blre.2019.04.005
Jayasuriya NA, Kjaergaard AD, Pedersen KM, Sørensen AL, Bak M, Larsen MK, Nordestgaard BG, Bojesen SE, Çolak Y, Skov V, Kjaer L, Tolstrup JS, Hasselbalch HC, Ellervik C (2020) Smoking, blood cells and myeloproliferative neoplasms: meta-analysis and Mendelian randomization of 2·3 million people. Br J Haematol 189(2):323–334. https://doi.org/10.1111/bjh.16321
Marneth AE, Mullally A (2020) The molecular genetics of myeloproliferative neoplasms. Cold Spring Harbor perspectives in medicine 10(2). https://doi.org/10.1101/cshperspect.a034876
Tefferi A, Guglielmelli P, Lasho TL, Coltro G, Finke CM, Loscocco GG, Sordi B, Szuber N, Rotunno G, Pacilli A, Hanson CA, Ketterling RP, Pardanani A, Gangat N, Vannucchi AM (2020) Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. British journal of haematology. doi:https://doi.org/10.1111/bjh.16380
Passamonti F, Elena C, Schnittger S, Skoda RC, Green AR, Girodon F, Kiladjian JJ, McMullin MF, Ruggeri M, Besses C, Vannucchi AM, Lippert E, Gisslinger H, Rumi E, Lehmann T, Ortmann CA, Pietra D, Pascutto C, Haferlach T, Cazzola M (2011) Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 117(10):2813–2816. https://doi.org/10.1182/blood-2010-11-316810
Tefferi A, Lavu S, Mudireddy M, Lasho TL, Finke CM, Gangat N, Pardanani A, Hanson CA, Mannarelli C, Guglielmelli P, Vannucchi AM (2018) JAK2 exon 12 mutated polycythemia vera: Mayo-Careggi MPN Alliance study of 33 consecutive cases and comparison with JAK2V617F mutated disease. Am J Hematol 93(4):E93–e96. https://doi.org/10.1002/ajh.25017
Pemmaraju N, Moliterno AR, Williams DM, Rogers O, Spivak JL (2007) The quantitative JAK2 V617F neutrophil allele burden does not correlate with thrombotic risk in essential thrombocytosis. Leukemia 21(10):2210–2212. https://doi.org/10.1038/sj.leu.2404755
Passamonti F, Randi ML, Rumi E, Pungolino E, Elena C, Pietra D, Scapin M, Arcaini L, Tezza F, Moratti R, Pascutto C, Fabris F, Morra E, Cazzola M, Lazzarino M (2007) Increased risk of pregnancy complications in patients with essential thrombocythemia carrying the JAK2 (617V>F) mutation. Blood 110(2):485–489. https://doi.org/10.1182/blood-2007-01-071068
Kiladjian JJ, Elkassar N, Cassinat B, Hetet G, Giraudier S, Balitrand N, Conejero C, Briere J, Fenaux P, Chomienne C, Grandchamp B (2006) Essential thrombocythemias without V617F JAK2 mutation are clonal hematopoietic stem cell disorders. Leukemia 20(6):1181–1183. https://doi.org/10.1038/sj.leu.2404214
Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, Skoda RC (2006) Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood 108(4):1377–1380. https://doi.org/10.1182/blood-2005-11-009605
Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S, Prchal JF, Prchal JT (2007) Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol 35(1):32–38. https://doi.org/10.1016/j.exphem.2006.11.012
Tefferi A, Lasho TL, Guglielmelli P, Finke CM, Rotunno G, Elala Y, Pacilli A, Hanson CA, Pancrazzi A, Ketterling RP, Mannarelli C, Barraco D, Fanelli T, Pardanani A, Gangat N, Vannucchi AM (2016) Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood advances 1(1):21–30. https://doi.org/10.1182/bloodadvances.2016000216
Karantanos T, Chaturvedi S, Braunstein EM, Spivak J, Resar L, Karanika S, Williams DM, Rogers O, Gocke CD, Moliterno AR (2020) Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood advances 4(12):2567–2576. https://doi.org/10.1182/bloodadvances.2019001407
Nielsen C, Birgens HS, Nordestgaard BG, Bojesen SE (2013) Diagnostic value of JAK2 V617F somatic mutation for myeloproliferative cancer in 49488 individuals from the general population. Br J Haematol 160(1):70–79. https://doi.org/10.1111/bjh.12099
Nielsen C, Bojesen SE, Nordestgaard BG, Kofoed KF, Birgens HS (2014) JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica 99(9):1448–1455. https://doi.org/10.3324/haematol.2014.107631
Spivak JL, Considine M, Williams DM, Talbot CC Jr, Rogers O, Moliterno AR, Jie C, Ochs MF (2014) Two clinical phenotypes in polycythemia vera. N Engl J Med 371(9):808–817. https://doi.org/10.1056/NEJMoa1403141
Barraco D, Cerquozzi S, Hanson CA, Ketterling RP, Pardanani AD, Gangat N, Tefferi A (2018) Cytogenetic findings in WHO-defined polycythaemia vera and their prognostic relevance. Br J Haematol 182(3):437–440. https://doi.org/10.1111/bjh.14798
Tang G, Hidalgo Lopez JE, Wang SA, Hu S, Ma J, Pierce S, Zuo W, Carballo-Zarate AA, Yin CC, Tang Z, Li S, Medeiros LJ, Verstovsek S, Bueso-Ramos CE (2017) Characteristics and clinical significance of cytogenetic abnormalities in polycythemia vera. Haematologica 102(9):1511–1518. https://doi.org/10.3324/haematol.2017.165795
Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, Guglielmelli P, Pancrazzi A, Salmoiraghi S, Zilio P, Ottomano C, Marchioli R, Cuccovillo I, Bottazzi B, Mantovani A, Rambaldi A (2011) Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica 96(2):315–318. https://doi.org/10.3324/haematol.2010.031070
Stein BL, Rademaker A, Spivak JL, Moliterno AR (2011) Gender and vascular complications in the JAK2 V617F-positive myeloproliferative neoplasms. Thrombosis 2011:874146. https://doi.org/10.1155/2011/874146
De Stefano V, Vannucchi AM, Ruggeri M, Cervantes F, Alvarez-Larran A, Iurlo A, Randi ML, Pieri L, Rossi E, Guglielmelli P, Betti S, Elli E, Finazzi MC, Finazzi G, Zetterberg E, Vianelli N, Gaidano G, Nichele I, Cattaneo D, Palova M, Ellis MH, Cacciola E, Tieghi A, Hernandez-Boluda JC, Pungolino E, Specchia G, Rapezzi D, Forcina A, Musolino C, Carobbio A, Griesshammer M, Barbui T (2016) Splanchnic vein thrombosis in myeloproliferative neoplasms: risk factors for recurrences in a cohort of 181 patients. Blood cancer journal 6(11):e493. https://doi.org/10.1038/bcj.2016.103
Lavu S, Szuber N, Mudireddy M, Yogarajah M, Gangat N, Pardanani A, Hanson CA, Ketterling RP, Ashrani AA, Kamath PS, Tefferi A (2018) Splanchnic vein thrombosis in patients with myeloproliferative neoplasms: the Mayo clinic experience with 84 consecutive cases. Am J Hematol 93(3):E61–e64. https://doi.org/10.1002/ajh.24993
Alvarez-Larran A, Pereira A, Magaz M, Hernandez-Boluda JC, Garrote M, Cuevas B, Ferrer-Marin F, Gomez-Casares MT, Garcia-Gutierrez V, Mata-Vazquez MI, Turon F, Hernandez-Gea V, Arellano-Rodrigo E, Cervantes F, Garcia-Pagan JC (2020) Natural history of polycythemia vera and essential thrombocythemia presenting with splanchnic vein thrombosis. Ann Hematol 99(4):791–798. https://doi.org/10.1007/s00277-020-03965-z
De Stefano V, Za T, Ciminello A, Betti S, Rossi E (2011) Causes of adult splanchnic vein thrombosis in the mediterranean area. Mediterranean journal of hematology and infectious diseases 3(1):e2011063. https://doi.org/10.4084/mjhid.2011.063
Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, Chamuleau RA, van Hattum J, Vleggaar FP, Hansen BE, Rosendaal FR, van Hoek B (2001) Extrahepatic portal vein thrombosis: aetiology and determinants of survival. Gut 49(5):720–724. https://doi.org/10.1136/gut.49.5.720
Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D (2000) Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology (Baltimore, Md) 31(3):587–591. https://doi.org/10.1002/hep.510310307
Valla D, Le MG, Poynard T, Zucman N, Rueff B, Benhamou JP (1986) Risk of hepatic vein thrombosis in relation to recent use of oral contraceptives. A case-control study. Gastroenterology 90(4):807–811. https://doi.org/10.1016/0016-5085(86)90855-3
Janssen HL, Meinardi JR, Vleggaar FP, van Uum SH, Haagsma EB, van Der Meer FJ, van Hattum J, Chamuleau RA, Adang RP, Vandenbroucke JP, van Hoek B, Rosendaal FR (2000) Factor V Leiden mutation, prothrombin gene mutation, and deficiencies in coagulation inhibitors associated with Budd-Chiari syndrome and portal vein thrombosis: results of a case-control study. Blood 96(7):2364–2368
Kiladjian JJ, Cervantes F, Leebeek FW, Marzac C, Cassinat B, Chevret S, Cazals-Hatem D, Plessier A, Garcia-Pagan JC, Darwish Murad S, Raffa S, Janssen HL, Gardin C, Cereja S, Tonetti C, Giraudier S, Condat B, Casadevall N, Fenaux P, Valla DC (2008) The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: a report on 241 cases. Blood 111(10):4922–4929. https://doi.org/10.1182/blood-2007-11-125328
Qi X, Ren W, De Stefano V, Fan D (2014) Associations of coagulation factor V Leiden and prothrombin G20210A mutations with Budd-Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 12 (11):1801–1812.e1807. doi:https://doi.org/10.1016/j.cgh.2014.04.026
Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, te Boekhorst PA, Commandeur S, Schouten HC, Sackmann F, Kerguelen Fuentes A, Hernandez-Maraver D, Pahl HL, Griesshammer M, Stegelmann F, Doehner K, Lehmann T, Bonatz K, Reiter A, Boyer F, Etienne G, Ianotto JC, Ranta D, Roy L, Cahn JY, Harrison CN, Radia D, Muxi P, Maldonado N, Besses C, Cervantes F, Johansson PL, Barbui T, Barosi G, Vannucchi AM, Passamonti F, Andreasson B, Ferrari ML, Rambaldi A, Samuelsson J, Birgegard G, Tefferi A, Mesa RA (2012) Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30(33):4098–4103. https://doi.org/10.1200/jco.2012.42.3863
Barbui T, Masciulli A, Marfisi MR, Tognoni G, Finazzi G, Rambaldi A, Vannucchi A (2015) White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood 126(4):560–561. https://doi.org/10.1182/blood-2015-04-638593
Vannucchi AM, Barbui T, Cervantes F, Harrison C, Kiladjian JJ, Kroger N, Thiele J, Buske C (2015) Philadelphia chromosome-negative chronic myeloproliferative neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 26(Suppl 5):v85–v99. https://doi.org/10.1093/annonc/mdv203
Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, Verstovsek S, Mesa R, Kiladjian JJ, Hehlmann R, Reiter A, Cervantes F, Harrison C, Mc Mullin MF, Hasselbalch HC, Koschmieder S, Marchetti M, Bacigalupo A, Finazzi G, Kroeger N, Griesshammer M, Birgegard G, Barosi G (2018) Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 32(5):1057–1069. https://doi.org/10.1038/s41375-018-0077-1
Pearson TC, Guthrie DL, Simpson J, Chinn S, Barosi G, Ferrant A, Lewis SM, Najean Y (1995) Interpretation of measured red cell mass and plasma volume in adults: expert panel on radionuclides of the International Council for Standardization in Haematology. Br J Haematol 89(4):748–756. https://doi.org/10.1111/j.1365-2141.1995.tb08411.x
Barbui T, Passamonti F, Accorsi P, Pane F, Vannucchi AM, Velati C, Gale RP, Tura S, Barosi G (2018) Evidence- and consensus-based recommendations for phlebotomy in polycythemia vera. Leukemia 32(9):2077–2081. https://doi.org/10.1038/s41375-018-0199-5
Kander EM, Moliterno AR, Rademaker A, Streiff MB, Spivak JL, Stein BL (2016) Practice patterns in the diagnosis and treatment of polycythemia vera in the post-JAK2 V617F discovery era. Journal of the National Comprehensive Cancer Network : JNCCN 14(10):1238–1245. https://doi.org/10.6004/jnccn.2016.0133
Grunwald MR, Kuter DJ, Altomare I, Burke JM, Gerds AT, Walshauser MA, Savona MR, Stein B, Oh ST, Colucci P, Parasuraman S, Paranagama D, Mesa R (2019) Treatment patterns and blood counts in patients with polycythemia vera treated with hydroxyurea in the United States: an analysis from the REVEAL study. Clinical lymphoma, myeloma & leukemia. https://doi.org/10.1016/j.clml.2019.09.601
Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch H, Kiladijan JJ, Lengfelder E, Mesa R, Mc Mullin MF, Passamonti F, Reilly JT, Vannucchi AM, Barbui T (2010) A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol 148(6):961–963. https://doi.org/10.1111/j.1365-2141.2009.08019.x
Alvarez-Larran A, Kerguelen A, Hernandez-Boluda JC, Perez-Encinas M, Ferrer-Marin F, Barez A, Martinez-Lopez J, Cuevas B, Mata MI, Garcia-Gutierrez V, Aragues P, Montesdeoca S, Burgaleta C, Caballero G, Hernandez-Rivas JA, Duran MA, Gomez-Casares MT, Besses C (2016) Frequency and prognostic value of resistance/intolerance to hydroxycarbamide in 890 patients with polycythaemia vera. Br J Haematol 172(5):786–793. https://doi.org/10.1111/bjh.13886
Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, Harrison CN, Pane F, Zachee P, Mesa R, He S, Jones MM, Garrett W, Li J, Pirron U, Habr D, Verstovsek S (2015) Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 372(5):426–435. https://doi.org/10.1056/NEJMoa1409002
Passamonti F, Griesshammer M, Palandri F, Egyed M, Benevolo G, Devos T, Callum J, Vannucchi AM, Sivgin S, Bensasson C, Khan M, Mounedji N, Saydam G (2017) Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. The Lancet Oncology 18(1):88–99. https://doi.org/10.1016/s1470-2045(16)30558-7
Verstovsek S, Vannucchi AM, Griesshammer M, Masszi T, Durrant S, Passamonti F, Harrison CN, Pane F, Zachee P, Kirito K, Besses C, Hino M, Moiraghi B, Miller CB, Cazzola M, Rosti V, Blau I, Mesa R, Jones MM, Zhen H, Li J, Francillard N, Habr D, Kiladjian JJ (2016) Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 101(7):821–829. https://doi.org/10.3324/haematol.2016.143644
Griesshammer M, Saydam G, Palandri F, Benevolo G, Egyed M, Callum J, Devos T, Sivgin S, Guglielmelli P, Bensasson C, Khan M, Ronco JP, Passamonti F (2018) Ruxolitinib for the treatment of inadequately controlled polycythemia vera without splenomegaly: 80-week follow-up from the RESPONSE-2 trial. Ann Hematol 97(9):1591–1600. https://doi.org/10.1007/s00277-018-3365-y
Tefferi A, Betti S, Barraco D, Mudireddy M, Shah S, Hanson CA, Ketterling RP, Pardanani A, Gangat N, Coltro G, Guglielmelli P, Vannucchi AM (2017) Gender and survival in essential thrombocythemia: a two-center study of 1494 patients. Am J Hematol 92(11):1193–1197. https://doi.org/10.1002/ajh.24882
Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, Orlandi E, Arcaini L, Brusamolino E, Pascutto C, Cazzola M, Morra E, Lazzarino M (2004) Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 117(10):755–761. https://doi.org/10.1016/j.amjmed.2004.06.032
Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, Gugliotta L, Landolfi R, Kutti J, Gisslinger H, Marilus R, Patrono C, Pogliani EM, Randi ML, Villegas A, Tognoni G, Barbui T (2005) Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 105(7):2664–2670. https://doi.org/10.1182/blood-2004-09-3426
Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, Randi ML, Vaidya R, Cazzola M, Rambaldi A, Gisslinger B, Pieri L, Ruggeri M, Bertozzi I, Sulai NH, Casetti I, Carobbio A, Jeryczynski G, Larson DR, Mullauer L, Pardanani A, Thiele J, Passamonti F, Barbui T (2013) Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 27(9):1874–1881. https://doi.org/10.1038/leu.2013.163
Alvarez-Larran A, Pereira A, Cervantes F, Arellano-Rodrigo E, Hernandez-Boluda JC, Ferrer-Marin F, Angona A, Gomez M, Muina B, Guillen H, Teruel A, Bellosillo B, Burgaleta C, Vicente V, Besses C (2012) Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 119(6):1363–1369. https://doi.org/10.1182/blood-2011-10-387787
Cerquozzi S, Tefferi A (2015) Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood cancer journal 5:e366. https://doi.org/10.1038/bcj.2015.95
Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, Levine R, Le Bousse-Kerdiles MC, Wadleigh M, Campbell PJ, Silver RT, Vannucchi AM, Deeg HJ, Gisslinger H, Thomas D, Odenike O, Solberg LA, Gotlib J, Hexner E, Nimer SD, Kantarjian H, Orazi A, Vardiman JW, Thiele J, Tefferi A (2007) Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 31(6):737–740. https://doi.org/10.1016/j.leukres.2006.12.002
Passamonti F, Mora B, Giorgino T, Guglielmelli P, Cazzola M, Maffioli M, Rambaldi A, Caramella M, Komrokji R, Gotlib J, Kiladjian JJ, Cervantes F, Devos T, Palandri F, De Stefano V, Ruggeri M, Silver R, Benevolo G, Albano F, Caramazza D, Rumi E, Merli M, Pietra D, Casalone R, Barbui T, Pieri L, Vannucchi AM (2017) Driver mutations’ effect in secondary myelofibrosis: an international multicenter study based on 781 patients. Leukemia 31(4):970–973. https://doi.org/10.1038/leu.2016.351
Yogarajah M, Tefferi A (2017) Leukemic transformation in myeloproliferative neoplasms: a literature review on risk, characteristics, and outcome. Mayo Clin Proc 92(7):1118–1128. https://doi.org/10.1016/j.mayocp.2017.05.010
Beer PA, Delhommeau F, LeCouedic JP, Dawson MA, Chen E, Bareford D, Kusec R, McMullin MF, Harrison CN, Vannucchi AM, Vainchenker W, Green AR (2010) Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood 115(14):2891–2900. https://doi.org/10.1182/blood-2009-08-236596
Kiladjian JJ, Chevret S, Dosquet C, Chomienne C, Rain JD (2011) Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol Off J Am Soc Clin Oncol 29(29):3907–3913. https://doi.org/10.1200/jco.2011.36.0792
Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, Marilus R, Villegas A, Tognoni G, Barbui T (2005) Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23(10):2224–2232. https://doi.org/10.1200/jco.2005.07.062
Bjorkholm M, Derolf AR, Hultcrantz M, Kristinsson SY, Ekstrand C, Goldin LR, Andreasson B, Birgegard G, Linder O, Malm C, Markevarn B, Nilsson L, Samuelsson J, Granath F, Landgren O (2011) Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29(17):2410–2415. https://doi.org/10.1200/jco.2011.34.7542
Bai J, Ai L, Zhang L, Yang FC, Zhou Y, Xue Y (2015) Incidence and risk factors for myelofibrotic transformation among 272 Chinese patients with JAK2-mutated polycythemia vera. Am J Hematol 90(12):1116–1121. https://doi.org/10.1002/ajh.24191
Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, Marfisi RM, Finazzi G, Guerini V, Fabris F, Randi ML, De Stefano V, Caberlon S, Tafuri A, Ruggeri M, Specchia G, Liso V, Rossi E, Pogliani E, Gugliotta L, Bosi A, Barbui T (2007) Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 110(3):840–846. https://doi.org/10.1182/blood-2006-12-064287
Bonora M, Wieckowsk MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34(12):1608. https://doi.org/10.1038/onc.2014.462
Cuthbert D, Stein BL (2019) Therapy-associated leukemic transformation in myeloproliferative neoplasms - what do we know? Best Pract Res Clin Haematol 32(1):65–73. https://doi.org/10.1016/j.beha.2019.02.004
Barraco D, Mora B, Guglielmelli P, Rumi E, Maffioli M, Rambaldi A, Caramella M, Komrokji R, Gotlib J, Kiladjian JJ, Cervantes F, Devos T, Palandri F, De Stefano V, Ruggeri M, Silver RT, Benevolo G, Albano F, Merli M, Pietra D, Barbui T, Rotunno G, Cazzola M, Giorgino T, Vannucchi AM, Passamonti F (2018) Gender effect on phenotype and genotype in patients with post-polycythemia vera and post-essential thrombocythemia myelofibrosis: results from the MYSEC project. Blood cancer journal 8(10):89. https://doi.org/10.1038/s41408-018-0128-x
Mora B, Giorgino T, Guglielmelli P, Rumi E, Maffioli M, Rambaldi A, Caramella M, Komrokji R, Gotlib J, Kiladjian JJ, Cervantes F, Devos T, Palandri F, De Stefano V, Ruggeri M, Silver RT, Benevolo G, Albano F, Cavalloni C, Barraco D, Merli M, Pietra D, Casalone R, Barbui T, Rotunno G, Cazzola M, Vannucchi AM, Passamonti F (2018) Value of cytogenetic abnormalities in post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a study of the MYSEC project. Haematologica 103(9):e392–e394. https://doi.org/10.3324/haematol.2017.185751
Robinson S, Bewley S, Hunt BJ, Radia DH, Harrison CN (2005) The management and outcome of 18 pregnancies in women with polycythemia vera. Haematologica 90(11):1477–1483
Alimam S, Bewley S, Chappell LC, Knight M, Seed P, Gray G, Harrison C, Robinson S (2016) Pregnancy outcomes in myeloproliferative neoplasms: UK prospective cohort study. Br J Haematol 175(1):31–36. https://doi.org/10.1111/bjh.14289
Bertozzi I, Rumi E, Cavalloni C, Cazzola M, Fabris F, Randi ML (2018) Pregnancy outcome and management of 25 pregnancies in women with polycythemia vera. Am J Hematol 93(9):E234–e235. https://doi.org/10.1002/ajh.25210
Maze M, Kazi S, Gupta V, Malinowski A, Fazelzad R, Shah P, Shehata N (2018) Pregnancy outcomes in patients with myeloproliferative neoplasms: a systematic review and meta-analysis. Blood 132:3046. https://doi.org/10.1182/blood-2018-99-118299
Elli EM, Diral E, Gambacorti-Passerini C, Calori R, Carmosino I, Breccia M, Latagliata R, Di Veroli A (2019) Management and outcome of 11 pregnancies in women with polycythemia vera. Leuk Res 81:25–26. https://doi.org/10.1016/j.leukres.2019.04.003
Gangat N, Wolanskyj AP, Schwager S, Tefferi A (2009) Predictors of pregnancy outcome in essential thrombocythemia: a single institution study of 63 pregnancies. Eur J Haematol 82(5):350–353. https://doi.org/10.1111/j.1600-0609.2009.01214.x
Rumi E, Bertozzi I, Casetti IC, Roncoroni E, Cavalloni C, Bellini M, Sant’Antonio E, Gotti M, Ferretti VV, Milanesi C, Peroni E, Pietra D, Astori C, Randi ML, Cazzola M (2015) Impact of mutational status on pregnancy outcome in patients with essential thrombocytemia. Haematologica 100(11):e443–e445. https://doi.org/10.3324/haematol.2015.131458
Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, Hehlmann R, Hoffman R, Kiladjian JJ, Kroger N, Mesa R, McMullin MF, Pardanani A, Passamonti F, Vannucchi AM, Reiter A, Silver RT, Verstovsek S, Tefferi A (2011) Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29(6):761–770. https://doi.org/10.1200/jco.2010.31.8436
Robinson SE, Harrison CN (2020) How we manage Philadelphia-negative myeloproliferative neoplasms in pregnancy. Br J Haematol. https://doi.org/10.1111/bjh.16453
Puyade M, Cayssials E, Pierre F, Pourrat O (2017) Pregnancy and myeloproliferative neoplasms : a retrospective monocentric cohort. Obstetric medicine 10(4):165–169. https://doi.org/10.1177/1753495x17708896
Griesshammer M, Struve S, Barbui T (2008) Management of Philadelphia negative chronic myeloproliferative disorders in pregnancy. Blood Rev 22(5):235–245. https://doi.org/10.1016/j.blre.2008.03.007
Beauverd Y, Radia D, Cargo C, Knapper S, Drummond M, Pillai A, Harrison C, Robinson S (2016) Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: outcome and safety. A case series. Haematologica 101(5):e182–e184. https://doi.org/10.3324/haematol.2015.139691
Perez-Encinas M, Bello JL, Perez-Crespo S, De Miguel R, Tome S (1994) Familial myeloproliferative syndrome. Am J Hematol 46(3):225–229. https://doi.org/10.1002/ajh.2830460312
Aliverti V, Bonanomi L, Giavini E (1980) Hydroxyurea as a reference standard in teratological screening. Comparison of the embryotoxic and teratogenic effects following single intraperitoneal or repeated oral administrations to pregnant rats. Archives of toxicology supplement = Archiv fur Toxikologie supplement 4:239-247
Wright CA, Tefferi A (2001) A single institutional experience with 43 pregnancies in essential thrombocythemia. Eur J Haematol 66(3):152–159. https://doi.org/10.1034/j.1600-0609.2001.00367.x
Datta P, Rewers-Felkins K, Kallem RR, Baker T, Hale TW (2017) Transfer of low dose aspirin into human milk. Journal of human lactation : official journal of International Lactation Consultant Association 33(2):296–299. https://doi.org/10.1177/0890334417695207
Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M (2018) Oral contraceptives and HRT risk of thrombosis. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis 24(2):217–225. https://doi.org/10.1177/1076029616683802
Pinkerton JV, James AH (2018) Management of menopausal symptoms for women who are at high risk of thrombosis. Clin Obstet Gynecol 61(2):260–268. https://doi.org/10.1097/grf.0000000000000358
Acknowledgments
We thank Dr. Daniela Bartoletti for her valuable technical assistance in the preparation and submission of the manuscript. We also thank Prof. Francesco Passamonti and Prof. Marco Ruggeri for stimulating and supporting this project.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palandri, F., Mora, B., Gangat, N. et al. Is there a gender effect in polycythemia vera?. Ann Hematol 100, 11–25 (2021). https://doi.org/10.1007/s00277-020-04287-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04287-w