Abstract
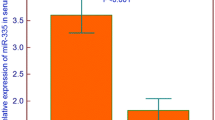
Although childhood acute lymphoblastic leukemia (ALL) is characterized by high remission rates, there are still patients who experience poor response to therapy or toxic effects due to intensive treatment. In the present study, we examined the expression profile of miR-143 and miR-182 in childhood ALL and evaluated their clinical significance for patients receiving Berlin–Frankfurt–Münster (BFM) protocol. Bone marrow specimens from 125 childhood ALL patients upon diagnosis and the end-of-induction (EoI; day 33), as well as from 64 healthy control children undergone RNA extraction, polyadenylation, and reverse transcription. Expression levels of miRNAs were quantified by qPCR analysis. Patients’ cytogenetic, immunohistotype and MRD evaluation was performed according to international guidelines. Median follow-up time was 86.0 months (95% CI 74.0–98.0), while patients’ mean DFS and OS intervals were 112.0 months (95% CI 104.2–119.8) and 109.2 months (95% CI 101.2–117.3), respectively. Bone marrow levels of miR-143/miR-182 were significantly decreased in childhood ALL patients at diagnosis and increased in more than 90% of patients at the EoI. Patients’ survival analysis highlighted that children overexpressing miR-143/miR-182 at the EoI presented significantly higher risk for short-term relapse (log-rank test: p = 0.021; Cox regression: HR = 4.911, p = 0.038) and death (log-rank test: p = 0.028; Cox regression: HR = 4.590, p = 0.046). Finally, the evaluation of the miR-143/miR-182 EoI levels along with the established disease prognostic markers resulted to improved prediction of BFM-treated patients’ survival outcome and response to therapy and additionally to superior BFM risk stratification specificity. Concluding, miR-143 and miR-182 could serve as novel prognostic molecular markers for pediatric ALL treated with BFM chemotherapy.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, Vora A, Baruchel A, Silverman LB, Schmiegelow K, Escherich G, Horibe K, Benoit YC, Izraeli S, Yeoh AE, Liang DC, Downing JR, Evans WE, Relling MV, Mullighan CG (2015) Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol 33(27):2938–2948. https://doi.org/10.1200/JCO.2014.59.1636
Pui C-H, Mullighan CG, Evans WE, Relling MV (2012) Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood 120(6):1165–1174. https://doi.org/10.1182/blood-2012-05-378943
Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig W-D, Ratei R, Harbott J, Boos J, Mann G, Niggli F, Feldges A, Henze G, Welte K, Beck J-D, Klingebiel T, Niemeyer C, Zintl F, Bode U, Urban C, Wehinger H, Niethammer D, Riehm H, Schrappe M (2008) Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111(9):4477–4489. https://doi.org/10.1182/blood-2007-09-112920
Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, Gonzalez A, Izraeli S, Janic D, Jazbec J, Konja J, Kaiserova E, Kowalczyk J, Kovacs G, Li C-K, Magyarosy E, Popa A, Stark B, Jabali Y, Trka J, Hrusak O, Riehm H, Masera G, Schrappe M (2014) Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the Randomized Intercontinental Trial ALL IC-BFM 2002. J Clin Oncol 32(3):174–184. https://doi.org/10.1200/jco.2013.48.6522
ALL IC-BFM 2009—a randomized trial of the I-BFM-SG for the management of childhood non-B acute lymphoblastic leukemia (2010) International BFM Study Group (I-BFM-SG), Kiel, Germany
Samudio I, Konopleva M, Carter B, Andreeff M (2010) Apoptosis in leukemias: regulation and therapeutic targeting. In: Nagarajan L (ed) Acute myelogenous leukemia: genetics, biology and therapy. Springer, New York, pp 197–217. https://doi.org/10.1007/978-0-387-69259-3_12
Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A (2012) The role of BCL2 family of apoptosis regulator proteins in acute and chronic Leukemias. Adv Hematol 2012:15. https://doi.org/10.1155/2012/524308
Vogler M, Walter HS, Dyer MJS (2017) Targeting anti-apoptotic BCL2 family proteins in haematological malignancies—from pathogenesis to treatment. Br J Haematol 178(3):364–379. https://doi.org/10.1111/bjh.14684
Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R (2004) Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ 11(S1):S45–S55
Groninger E, Meeuwsen-De Boer GJ, De Graaf SS, Kamps WA, De Bont ES (2002) Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: a mitochondrial controlled pathway regulated by reactive oxygen species? Int J Oncol 21(6):1339–1345. https://doi.org/10.3892/ijo.21.6.1339
Richardson DS, Johnson SA (1997) Anthracyclines in haematology: preclinical studies, toxicity and delivery systems. Blood Rev 11(4):201–223. https://doi.org/10.1016/S0268-960X(97)90020-5
Fransecky L, Mochmann LH, Baldus CD (2015) Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell Ther 3:2. https://doi.org/10.1186/s40591-015-0040-8
Fuka G, Kantner HP, Grausenburger R, Inthal A, Bauer E, Krapf G, Kaindl U, Kauer M, Dworzak MN, Stoiber D, Haas OA, Panzer-Grumayer R (2012) Silencing of ETV6/RUNX1 abrogates PI3K/AKT/mTOR signaling and impairs reconstitution of leukemia in xenografts. Leukemia 26(5):927–933 http://www.nature.com/leu/journal/v26/n5/suppinfo/leu2011322s1.html
Silva A, Girio A, Cebola I, Santos CI, Antunes F, Barata JT (2011) Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia 25(6):960–967 http://www.nature.com/leu/journal/v25/n6/suppinfo/leu201156s1.html
Hers I, Vincent EE, Tavaré JM (2011) Akt signalling in health and disease. Cell Signal 23(10):1515–1527. https://doi.org/10.1016/j.cellsig.2011.05.004
Martelli AM, Evangelisti C, Chappell W, Abrams SL, Basecke J, Stivala F, Donia M, Fagone P, Nicoletti F, Libra M, Ruvolo V, Ruvolo P, Kempf CR, Steelman LS, McCubrey JA (2011) Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia 25(7):1064–1079
Morishita N, Tsukahara H, Chayama K, Ishida T, Washio K, Miyamura T, Yamashita N, Oda M, Morishima T (2012) Activation of Akt is associated with poor prognosis and chemotherapeutic resistance in pediatric B-precursor acute lymphoblastic leukemia. Pediatr Blood Cancer 59(1):83–89. https://doi.org/10.1002/pbc.24034
Bissels U, Bosio A, Wagner W (2012) MicroRNAs are shaping the hematopoietic landscape. Haematologica 97(2):160–167. https://doi.org/10.3324/haematol.2011.051730
Zhao H, Wang D, Du W, Gu D, Yang R (2010) MicroRNA and leukemia: tiny molecule, great function. Crit Rev Oncol Hematol 74(3):149–155. https://doi.org/10.1016/j.critrevonc.2009.05.001
Schotte D, Chau JC, Sylvester G, Liu G, Chen C, van der Velden VH, Broekhuis MJ, Peters TC, Pieters R, den Boer ML (2009) Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia 23(2):313–322. https://doi.org/10.1038/leu.2008.286
Wang Y, Li Z, He C, Wang D, Yuan X, Chen J, Jin J (2010) MicroRNAs expression signatures are associated with lineage and survival in acute leukemias. Blood Cell Mol Dis 44(3):191–197. https://doi.org/10.1016/j.bcmd.2009.12.010
Schotte D, De Menezes RX, Moqadam FA, Khankahdani LM, Lange-Turenhout E, Chen C, Pieters R, Den Boer ML (2011) MicroRNA characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica 96(5):703–711. https://doi.org/10.3324/haematol.2010.026138
Schotte D, Pieters R, Den Boer ML (2012) MicroRNAs in acute leukemia: from biological players to clinical contributors. Leukemia 26(1):1–12. https://doi.org/10.1038/leu.2011.151
Zhang H, Cai X, Wang Y, Tang H, Tong D, Ji F (2010) microRNA-143, down-regulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol Rep 24(5):1363–1369. https://doi.org/10.3892/or_00000994
Yan D, Dong XD, Chen X, Yao S, Wang L, Wang J, Wang C, Hu D-N, Qu J, Tu L (2012) Role of microRNA-182 in posterior uveal melanoma: regulation of tumor development through MITF, BCL2 and cyclin D2. PLoS One 7(7):e40967. https://doi.org/10.1371/journal.pone.0040967
Noguchi S, Yasui Y, Iwasaki J, Kumazaki M, Yamada N, Naito S, Akao Y (2013) Replacement treatment with microRNA-143 and -145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett 328(2):353–361. https://doi.org/10.1016/j.canlet.2012.10.017
Guttilla IK, White BA (2009) Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem 284(35):23204–23216. https://doi.org/10.1074/jbc.M109.031427
Yang A, Ma J, Wu M, Qin W, Zhao B, Shi Y, Jin Y, Xie Y (2012) Aberrant microRNA-182 expression is associated with glucocorticoid resistance in lymphoblastic malignancies. Leuk Lymphoma 53(12):2465–2473. https://doi.org/10.3109/10428194.2012.693178
Pui C-H (2010) Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc 109(11):777–787. https://doi.org/10.1016/S0929-6646(10)60123-4
Bhojwani D, Pui C-H (2013) Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol 14(6):e205–e217. https://doi.org/10.1016/S1470-2045(12)70580-6
de Oliveira JC, Brassesco MS, Scrideli CA, Tone LG, Narendran A (2012) MicroRNA expression and activity in pediatric acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer 59(4):599–604. https://doi.org/10.1002/pbc.24167
Zhang H, Luo X-Q, Zhang P, Huang L-B, Zheng Y-S, Wu J, Zhou H, Qu L-H, Xu L, Chen Y-Q (2009) MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS One 4(11):e7826. https://doi.org/10.1371/journal.pone.0007826
Zhang H, Yang J-H, Zheng Y-S, Zhang P, Chen X, Wu J, Xu L, Luo X-Q, Ke Z-Y, Zhou H, Qu L-H, Chen Y-Q (2009) Genome-wide analysis of small RNA and novel microRNA discovery in human acute lymphoblastic leukemia based on extensive sequencing approach. PLoS One 4(9):e6849. https://doi.org/10.1371/journal.pone.0006849
Han B-W, Feng D-D, Li Z-G, Luo X-Q, Zhang H, Li X-J, Zhang X-J, Zheng L-L, Zeng C-W, Lin K-Y, Zhang P, Xu L, Chen Y-Q (2011) A set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Hum Mol Genet 20(24):4903–4915. https://doi.org/10.1093/hmg/ddr428
Shen J, Zhang Y, Fu H, Wu D, Zhou H (2014) Overexpression of microRNA-143 inhibits growth and induces apoptosis in human leukemia cells. Oncol Rep 31(5):2035–2042. https://doi.org/10.3892/or.2014.3078
Dou L, Zheng D, Li J, Li Y, Gao L, Wang L, Yu L (2012) Methylation-mediated repression of microRNA-143 enhances MLL-AF4 oncogene expression. Oncogene 31(4):507–517 http://www.nature.com/onc/journal/v31/n4/suppinfo/onc2011248s1.html
dos Santos Ferreira AC, Robaina MC, de Rezende LMM, Severino P, Klumb CE (2014) Histone deacetylase inhibitor prevents cell growth in Burkitt’s lymphoma by regulating PI3K/Akt pathways and leads to upregulation of miR-143, miR-145, and miR-101. Ann Hematol 93(6):983–993. https://doi.org/10.1007/s00277-014-2021-4
Akao Y, Nakagawa Y, Iio A, Naoe T (2009) Role of microRNA-143 in Fas-mediated apoptosis in human T-cell leukemia Jurkat cells. Leuk Res 33(11):1530–1538. https://doi.org/10.1016/j.leukres.2009.04.019
Akao Y, Nakagawa Y, Kitade Y, Kinoshita T, Naoe T (2007) Downregulation of microRNAs-143 and -145 in B-cell malignancies. Cancer Sci 98(12):1914–1920. https://doi.org/10.1111/j.1349-7006.2007.00618.x
Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y (2009) Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology 77(1):12–21
Noguchi S, Mori T, Hoshino Y, Maruo K, Yamada N, Kitade Y, Naoe T, Akao Y (2011) MicroRNA-143 functions as a tumor suppressor in human bladder cancer T24 cells. Cancer Lett 307(2):211–220. https://doi.org/10.1016/j.canlet.2011.04.005
Dixon-McIver A, East P, Mein CA, Cazier J-B, Molloy G, Chaplin T, Andrew Lister T, Young BD, Debernardi S (2008) Distinctive patterns of microRNA expression associated with karyotype in acute myeloid leukaemia. PLoS One 3(5):e2141. https://doi.org/10.1371/journal.pone.0002141
Lai T-H, Zecevic A, Ewald B, Chaomei L, Rizzotto L, Sulda M, Papaioannou D, Garzon R, Plunkett W, Sampath D (2015) HDAC inhibition induces microRNA-182 which targets Rad51 protein and impairs homologous recombination repair to sensitize cells to the double strand break inducing nucleoside analog, sapacitabine in AML. Blood 126(23):3639–3639
Sun Y, Fang R, Li C, Li L, Li F, Ye X, Chen H (2010) Hsa-Mir-182 suppresses lung tumorigenesis through down regulation of RGS17 expression in vitro. Biochem Biophys Res Commun 396(2):501–507. https://doi.org/10.1016/j.bbrc.2010.04.127
Kong W-Q, Bai R, Liu T, Cai C-L, Liu M, Li X, Tang H (2012) MicroRNA-182 targets cAMP-responsive element-binding protein 1 and suppresses cell growth in human gastric adenocarcinoma. FEBS J 279(7):1252–1260. https://doi.org/10.1111/j.1742-4658.2012.08519.x
Wurm AA, Zjablovskaja P, Kardosova M, Gerloff D, Brauer-Hartmann D, Katzerke C, Hartmann JU, Benoukraf T, Fricke S, Hilger N, Muller AM, Bill M, Schwind S, Tenen DG, Niederwieser D, Alberich-Jorda M, Behre G (2017) Disruption of the C/EBPalpha-miR-182 balance impairs granulocytic differentiation. Nat Commun 8(1):46. https://doi.org/10.1038/s41467-017-00032-6
Cheng T, Hu C, Yang H, Cao L, An J (2014) Transforming growth factor-β-induced miR-143 expression in regulation of non-small cell lung cancer cell viability and invasion capacity in vitro and in vivo. Int J Oncol 45(5):1977–1988. https://doi.org/10.3892/ijo.2014.2623
Blank U, Karlsson S (2011) The role of Smad signaling in hematopoiesis and translational hematology. Leukemia 25(9):1379–1388. https://doi.org/10.1038/leu.2011.95
Larsson J, Karlsson S (2005) The role of Smad signaling in hematopoiesis. Oncogene 24(37):5676–5692. https://doi.org/10.1038/sj.onc.1208920
Ma J, Xie Y, Shi Y, Qin W, Zhao B, Jin Y (2008) Glucocorticoid-induced apoptosis requires FOXO3A activity. Biochem Biophys Res Commun 377(3):894–898. https://doi.org/10.1016/j.bbrc.2008.10.097
Fasihi-Ramandi M, Moridnia A, Najafi A, Sharifi M (2017) Inducing cell proliferative prevention in human acute promyelocytic leukemia by miR-182 inhibition through modulation of CASP9 expression. Biomed Pharmacother 89:1152–1158. https://doi.org/10.1016/j.biopha.2017.02.100
Sharifi M, Moridnia A (2017) Apoptosis-inducing and antiproliferative effect by inhibition of miR-182-5p through the regulation of CASP9 expression in human breast cancer. Cancer Gene Ther 24(2):75–82. https://doi.org/10.1038/cgt.2016.79
Jiang L, Mao P, Song L, Wu J, Huang J, Lin C, Yuan J, Qu L, Cheng S-Y, Li J (2010) miR-182 as a prognostic marker for glioma progression and patient survival. Am J Pathol 177(1):29–38. https://doi.org/10.2353/ajpath.2010.090812
Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R (2013) MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One 8(1):e55502. https://doi.org/10.1371/journal.pone.0055502
Song C, Zhang L, Wang J, Huang Z, Li X, Wu M, Li S, Tang H, Xie X (2016) High expression of microRNA-183/182/96 cluster as a prognostic biomarker for breast cancer. Sci Rep 6:24502. https://doi.org/10.1038/srep24502
Kulda V, Pesta M, Topolcan O, Liska V, Treska V, Sutnar A, Rupert K, Ludvikova M, Babuska V, Holubec L, Cerny R (2010) Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet 200(2):154–160. https://doi.org/10.1016/j.cancergencyto.2010.04.015
Avgeris M, Mavridis K, Tokas T, Stravodimos K, Fragoulis EG, Scorilas A (2015) Uncovering the clinical utility of miR-143, miR-145 and miR-224 for predicting the survival of bladder cancer patients following treatment. Carcinogenesis 36(5):528–537. https://doi.org/10.1093/carcin/bgv024
Akagi I, Miyashita M, Ishibashi O, Mishima T, Kikuchi K, Makino H, Nomura T, Hagiwara N, Uchida E, Takizawa T (2011) Relationship between altered expression levels of MIR21, MIR143, MIR145, and MIR205 and clinicopathologic features of esophageal squamous cell carcinoma. Dis Esophagus 24(7):523–530. https://doi.org/10.1111/j.1442-2050.2011.01177.x
Acknowledgments
We wish to sincerely thank Dr. M. Varvoutsi, Dr. D. Doganis, and Dr. M. Servitzoglou for their valuable professional assistance in the characterization and collection of our samples. We would also like to thank the nursing staff of the Department of Pediatric Oncology, “P. & A. Kyriakou” Children’s Hospital, for their expert help with the collection of samples.
Author information
Authors and Affiliations
Contributions
Conception and design: D. Gourgiotis, A. Scorilas, M. Avgeris
Development of methodology: M. Avgeris, D. Piatopoulou, M. Xagorari
Acquisition of data: D. Piatopoulou, M. Avgeris, I. Drakaki, A. Marmarinos, M. Xagorari, M. Baka, A. Pourtsidis, L. Kossiva
Analysis and interpretation of data: M. Avgeris, D. Piatopoulou
Acquired and managed patients: M. Baka, A. Pourtsidis, L. Kossiva
Drafting of the manuscript: D. Piatopoulou, M. Avgeris, I. Drakaki, A. Marmarinos, M. Xagorari
Critical revision of the manuscript: M. Avgeris, A. Marmarinos, M. Baka, A. Pourtsidis, L. Kossiva, D. Gourgiotis, A. Scorilas
Administrative, technical, or material support: D. Gourgiotis, A. Scorilas, M. Baka, A. Pourtsidis, L. Kossiva
Study supervision: A. Scorilas, D. Gourgiotis
Approval of the submitted and final version: all authors
Corresponding author
Ethics declarations
The study was approved by the Ethics Committee of “P. & A. Kyriakou” Children’s Hospital, Athens, Greece, and performed with respect to the ethical standards of the Declaration of Helsinki, as revised in 2008. Informed consent was obtained from all parents and legal guardians of the participating patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Table 1
(DOCX 15 kb)
Supplemental Table 2
(DOC 42 kb)
Supplemental Table 3
(DOC 41 kb)
Supplemental Table 4
(DOCX 12 kb)
Supplemental Table 5
(DOCX 13 kb)
Supplemental Table 6
(DOCX 20 kb)
Supplemental Table 7
(DOCX 20 kb)
Supplemental Fig. 1
(GIF 195 kb)
Supplemental Fig. 2
(GIF 193 kb)
Supplemental Fig. 3
(GIF 256 kb)
Rights and permissions
About this article
Cite this article
Piatopoulou, D., Avgeris, M., Drakaki, I. et al. Clinical utility of miR-143/miR-182 levels in prognosis and risk stratification specificity of BFM-treated childhood acute lymphoblastic leukemia. Ann Hematol 97, 1169–1182 (2018). https://doi.org/10.1007/s00277-018-3292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3292-y