Abstract
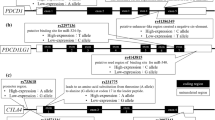
Single-nucleotide polymorphisms (SNPs) of cytotoxic T lymphocyte antigen-4 (CTLA-4) are important risk factors associated with autoimmune diseases and malignancies. This study explored the association of CTLA-4SNPs with the development of myeloma and evaluated the outcome of patients receiving bortezomib-based regimens in relation to CTLA-4SNPs. Peripheral blood samples from 86 patients with multiple myeloma (MM) and 154 healthy controls were obtained to investigate CTLA4 polymorphisms. Five SNP genotypes of CTLA-4, namely, −1772 (rs733618), −1661 (rs4553808), −318 (rs5742909), CT60 (rs3087243), and +49 (rs231775), were evaluated through TaqMan SNP genotyping assays (Applied Biosystems). Some of the CTLA-4 polymorphisms displayed frequencies that vary among ethnic groups. Kaplan–Meier analysis revealed that patients with rs733618 GG showed a significantly lower disease-free survival (0 vs. 57.4%, P = 0.020) and overall survival (46.3 vs. 83.3%, P = 0.026) than those with GA+AA following bortezomib-based therapy. Multivariate analyses showed that rs733618 GG was a risk factor for OS (HR = 0.012; 95% CI = 0.001–0.199; P = 0.002). The incidence of nonhematologic grade 3/4 adverse events significantly increased in the rs4553808 GG+GA group compared with that in the AA group (P = 0.036). CTLA-4 rs733618 GG reduced the progression-free survival and the overall survival of patients with MM who received bortezomib-based therapy. Information regarding CTLA-4 polymorphisms and haplotypes may be used to improve MM therapy. Future studies must determine the precise effect of CTLA-4 polymorphisms and haplotypes on MM therapy outcomes by using different cohorts with a large number of subjects.


Similar content being viewed by others
References
Kyle RA, Rajkumar SV (2004) Multiple myeloma. N Engl J Med 351(18):1860–1873
Richardson PG, Barlogie B, Berenson J et al (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348(26):2609–2617
Richardson PG, Sonneveld P, Schuster MW et al (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352(24):2487–2498
San Miguel JF, Schlag R, Khuageva NK et al (2008) Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 359(9):906–917
Orlowski RZ, Kuhn DJ (2008) Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res 14(6):1649–1657
Pineda-Roman M, Zangari M, Haessler J et al (2008) Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol 140(6):625–634
Rajkumar SV, Sonneveld P (2009) Front-line treatment in younger patients with multiple myeloma. Semin Hematol 46(2):118–126
Teft WA, Kirchhof MG, Madrenas J (2006) A molecular perspective of CTLA-4 function. Annu Rev Immunol 24:65–97
Ahmed S, Ihara K, Kanemitsu S et al (2001) Association of CTLA-4 but not CD28 gene polymorphisms with systemic lupus erythematosus in the Japanese population. Rheumatology (Oxford) 40:662–667
Hudson LL, Rocca K, Song YW et al (2002) CTLA-4 gene polymorphisms in systemic lupus erythematosus: a highly significant association with a determinant in the promoter region. Hum Genet 111:452–455
Haller K, Kisand K, Pisarev H et al (2007) Insulin gene VNTR, CTLA-4 +49A/G and HLA-DQB1 alleles distinguish latent autoimmune diabetes in adults from type 1 diabetes and from type 2 diabetes group. Tissue Antigens 69:121–127
Balic I, Angel B, Codner E et al (2009) Association of CTLA-4 polymorphisms and clinical-immunologic characteristics at onset of type 1 diabetes mellitus in children. Hum Immunol 70:116–120
Kouki T, Sawai Y, Gardine CA et al (2000) CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves’ disease. J Immunol 165:6606–6611
Sun T, Zhou Y, Yang M et al (2008) Functional genetic variations in cytotoxic T-lymphocyte antigen 4 and susceptibility to multiple types of cancer. Cancer Res 68:7025–7034
Ghaderi A, Yeganeh F, Kalantari T et al (2004) Cytotoxic T lymphocyte antigen-4 gene in breast cancer. Breast Cancer Res Treat 86:1–7
Wang L, Li D, Fu Z et al (2007) Association of CTLA-4 gene polymorphisms with sporadic breast cancer in Chinese Han population. BMC Cancer 7:173
Li D, Zhang Q, Xu F et al (2012) Association of CTLA-4 gene polymorphisms with sporadic breast cancer risk and clinical features in Han women of northeast china. Mol Cell Biochem 364:283–290
Su TH, Chang TY, Lee YJ et al (2007) CTLA-4 gene and susceptibility to human papillomavirus-16-associated cervical squamous cell carcinoma in Taiwanese women. Carcinogenesis 28:1237–1240
Castro FA, Haimila K, Sareneva I et al (2009) Association of HLA-DRB1, interleukin-6 and cyclin D1 polymorphisms with cervical cancer in the Swedish population—a candidate gene approach. Int J Cancer 125:1851–1858
Pawlak E, Karabon L, Wlodarska-Polinska I et al (2010) Influence of CTLA-4/CD28/ICOS gene polymorphisms on the susceptibility to cervical squamous cell carcinoma and stage of differentiation in the Polish population. Hum Immunol 71:195–200
Ivansson EL, Juko-Pecirep I, Gyllensten UB (2010) Interaction of immunological genes on chromosome 2q33 and IFNG in susceptibility to cervical cancer. Gynecol Oncol 116:544–548
Rahimifar S, Erfani N, Sarraf Z et al (2010) CTLA-4 gene variations may influence cervical cancer susceptibility. Gynecol Oncol 119:136–139
Li H, Zhou YF, Guo HY et al (2011) Association between CTLA-4 gene polymorphism and susceptibility to cervical cancer. Zhonghua Zhong Liu Za Zhi 33:681–684
Jiang L, Luo RY, Zhang W et al (2011) Single nucleotide polymorphisms of CTLA4 gene and their association with human cervical cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 28:313–317
Gokhale P, Kerkar S, Tongaonkar H et al (2013) CTLA-4 gene polymorphism at position +49 A>G in exon 1: a risk factor for cervical cancer in Indian women. Cancer Genet 206:154–161
Khaghanzadeh N, Erfani N, Ghayumi MA et al (2010) CTLA4 gene variations and haplotypes in patients with lung cancer. Cancer Genet Cytogenet 196:171–174
Song B, Liu Y, Liu J et al (2011) CTLA-4 +49A>G polymorphism is associated with advanced non-small cell lung cancer prognosis. Respiration 82:439–444
Karabon L, Pawlak E, Tomkiewicz A et al (2011) CTLA-4, CD28, and ICOS gene polymorphism associations with non-small-cell lung cancer. Hum Immunol 72:947–954
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736
Blade J, Samson D, Reece D et al (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 102:1115–1123
Durie BG, Harousseau JL, Miguel JS et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473
National Cancer Institute. National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, Version 3.0. 9 August 2006.http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30
Hideshima T, Ikeda H, Chauhan D et al (2009) Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood 114(5):1046–1052
Richardson PG, Laubach J, Mitsiades C et al (2010) Tailoring treatment for multiple myeloma patients with relapsed and refractory disease. Oncology (Williston Park) 24(3 Suppl 2):22–29
Bretscher P, Cohn M (1970) A theory of self-nonself discrimination. Science 267:1485–1488
Mueller DL, Jenkins MK, Swartz RH (1989) An accessory cell-derived co-stimulatory signal acts independently of protein kinase C activation to allow T cell proliferation and prevent the induction of unresponsiveness. J Immunol 142:2617–2628
Harding FA, McArthur JG, Raulet DH et al (1992) CD28-mediated signaling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356:607–609
Raab M, Cai YC, Bunnell SC et al (1995) p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc Natl Acad Sci U S A 92:8891–8895
Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441(7092):431–436
Schwartz RH (2003) T cell anergy. Annu Rev Immunol 21:305–334
Krummel MF, Allison JP (1996) CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183:2533–2540
Purohit S, Podolsky R, Collins C et al (2005) Lack of correlation between the levels of soluble cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and the CT-60 genotypes. J Autoimmune Dis 2:8
Liu MF, Wang CR, Chen PC et al (2003) Increased expression of soluble cytotoxic T-lymphocyte associated antigen-4 molecule in patients with systemic lupus erythematosus. Scand J Immunol 57:568–572
Sato S, Fujimoto M, Hasegawa M et al (2004) Serum soluble CTLA-4 levels are increased in diffuse cutaneous systemic sclerosis. Rheumatology (Oxford) 43:1261–1266
Wong CK, Lit LC, Tam LS et al (2005) Aberrant production of soluble costimulatory molecules CTLA-4,CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 44:989–994
Oaks MK, Hallett KM, Penwell RT et al (2000) A native soluble form of CTLA-4. Cell Immunol 201:144–153
Saverino D, Brizzolara R, Simone R et al (2007) Soluble CTLA-4 in autoimmune thyroid diseases: relationship with clinical status and possible role in the immune response dysregulation. Clin Immunol 123:190–198
Perez-Garcia A, De la Camara R, Roman-Gomez J et al (2007) CTLA-4 polymorphisms and clinical outcome after allogeneic stem cell transplantation from HLA-identical sibling donors. Blood 110:461–467
Ueda H, Howson JM, Esposito L et al (2003) Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423:506–511
Mäurer M, Loserth S, Kolb-Mäurer A et al (2002) A polymorphism in the human cytotoxic T-lymphocyte antigen 4 (CTLA4) gene (exon 1 +49) alters T-cell activation. Immunogenetics 54:1–8
Ligers A, Teleshova N, Masterman T et al (2001) CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun 2:145–152
Wang XB, Zhao X, Giscombe R et al (2002) A CTLA-4 gene polymorphism at position -318 in the promoter region affects the expression of protein. Genes Immun 3:233–234
Yousefipour G, Erfani N, Momtahan M et al (2009) CTLA4 exon 1 and promoter polymorphisms in patients with multiple sclerosis. Acta Neurol Scand 120:424–429
Baniasadi V, Narain N, Goswami R et al (2006) Promoter region 2318 C/T and 21661 A/G CTLA-4 single nucleotide polymorphisms and type 1 diabetes in North Indians. Tissue Antigens 67:383–389
Bouqbis L, Izaabel H, Akhayat O, Pérez-Lezaun A et al (2003) Association of the CTLA4 promoter region (−1661G allele) with type 1 diabetes in the South Moroccan population. Genes Immun 4:132–137
Almasi S, Erfani N, Mojtahedi Z et al (2006) Association of CTLA-4 gene promoter polymorphisms with systemic sclerosis in Iranian population. Genes Immun 7:401–406
Kammerer PW, Toyoshima T, Schoder F et al (2010) Association of T-cell regulatory gene polymorphisms with oral squamous cell carcinoma. Oral Oncol 46:543–548
Erfani N, Razmkhah M, Talei AR et al (2006) Cytotoxic T lymphocyte antigen-4 promoter variants in breast cancer. Cancer Genet Cytogenet 165:114–120
Bharti V, Mohanti BK, Das SN (2013) Functional genetic variants of CTLA-4 and risk of tobacco-related oral carcinoma in high-risk north Indian population. Hum Immunol 74:348–352
Fernandez-Blanco L, Perez-Pampin E, Gomez-Reino JJ et al (2004) A CTLA-4 polymorphism associated with susceptibility to systemic lupus erythematosus. Arthritis Rheum 50(1):328–329
Liu J, Zhang H (2013 Mar) -1722T/C polymorphism (rs733618) of CTLA-4 significantly associated with systemic lupus erythematosus (SLE): a comprehensive meta-analysis. Hum Immunol 74(3):341–347
Mateos MV, Oriol A, Martinez J, et al. (2009) A prospective, multicenter, randomized, trial of bortezomib/melphalan/prednisone (VMP) versus bortezomib/thalidomide/prednisone (VTP) as induction therapy Followed by maintenance treatment with bortezomib/thalidomide (VT) versus bortezomib/prednisone (VP) in elderly untreated patients with multiple myeloma older than 65 years. Blood (ASH Annual Meeting Abstracts). 114(22):Abstract 3
Reece DE, Trieu Y, Chen C, et al. (2009) Sequencing novel agents in relapsed/refractory multiple myeloma:use of bortezomib-based therapy after lenalidomide + dexamethasone. Blood (ASH Annual Meeting Abstracts). 114(22):Abstract 1853
Mao HT, Wang XB, Zhang L et al (2004) Studies on the genetic pathogenesis of myasthenia gravis caused by CTLA-4 gene polymorphism. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 21:574–578
Jones KA, Kadonaga JT, Rosenfeld PJ et al (1987) A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell 48:79–89
Ahmadzadeh M, Johnson LA, Heemskerk B et al (2009 Aug 20) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114(8):1537–1544
Marshall NA, Christie LE, Munro LR et al (2004) Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103(5):1755–1762
Brown RD, Pope B, Yuen E et al (1998) The expression of T cell related costimulatory molecules in multiple myeloma. Leuk Lymphoma 31(3):379–384
Richardson PG, Sonneveld P, Schuster MW et al (2009) Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol 144:895–903
Favis R, Sun Y, van de Velde H et al (2011 Mar) Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharmacogenet Genomics 21(3):121–129
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Richardson PG, Barlogie B, Berenson J et al (2005 Nov 1) Clinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myeloma. Blood 106(9):2977–2981
Acknowledgements
We are grateful to all colleagues for their participation in this research.
Funding
This work was supported by the Key Program of the National Natural Science Foundation of China (81230013, 81530046), the Innovative Research Groups of the National Natural Science Foundation of China (81621001), the National Natural Science Foundation of China (grants 81372535 and 81670192), the Beijing Municipal Science and Technology Commission (no. Z121107002612035), the Scientific Research Foundation for Capital Medicine Development (2011-4022-08), and the China National Science and Technology Major Project during12th Five-Year Plan Period (2012ZX09303019).
Author information
Authors and Affiliations
Contributions
XYQ conducted molecular genetic studies. XYQ and JL collected and analyzed data and wrote the manuscript. GXL, LW, YL, LPX, YJC, KYL, and ZFJ collected and interpreted the data. XJH designed the study and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients provided informed consent for treatment, which was performed using a protocol reviewed and approved by the Peking University Institute of Hematology. All healthy individuals also provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Qin, XY., Lu, J., Li, GX. et al. CTLA-4 polymorphisms are associated with treatment outcomes of patients with multiple myeloma receiving bortezomib-based regimens. Ann Hematol 97, 485–495 (2018). https://doi.org/10.1007/s00277-017-3203-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3203-7